Page 313 - Biomedical Engineering and Design Handbook Volume 1, Fundamentals
P. 313
290 BIOMECHANICS OF THE HUMAN BODY
the synaptic cleft. Acetylcholine diffuses across the synaptic cleft and binds to the nicotinic acetyl-
+
+
choline receptors of a transmitter-gated Na -K channel. When bounded by the acetylcholine, the
+
+
+
+
channel is open, and Na flows into the muscle cell and K flows out. The flow of Na and K gener-
ates a local depolarization of the motor end plate, known as end-plate potential. This depolarization
spreads and further triggers a sarcolemmal action potential from the sarcolemma in the region of the
neuromuscular junction.
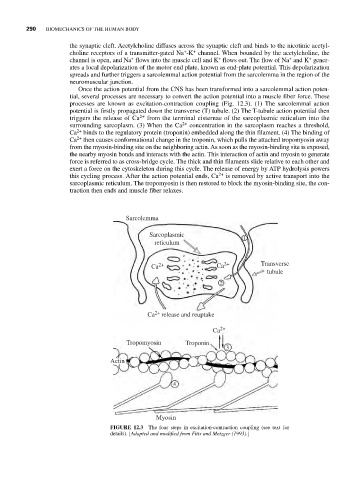
Once the action potential from the CNS has been transformed into a sarcolemmal action poten-
tial, several processes are necessary to convert the action potential into a muscle fiber force. These
processes are known as excitation-contraction coupling (Fig. 12.3). (1) The sarcolemmal action
potential is firstly propagated down the transverse (T) tubule. (2) The T-tubule action potential then
triggers the release of Ca 2+ from the terminal cisternae of the sarcoplasmic reticulum into the
surrounding sarcoplasm. (3) When the Ca 2+ concentration in the sarcoplasm reaches a threshold,
2+
Ca binds to the regulatory protein (troponin) embedded along the thin filament. (4) The binding of
2+
Ca then causes conformational change in the troponin, which pulls the attached tropomyosin away
from the myosin-binding site on the neighboring actin. As soon as the myosin-binding site is exposed,
the nearby myosin bonds and interacts with the actin. This interaction of actin and myosin to generate
force is referred to as cross-bridge cycle. The thick and thin filaments slide relative to each other and
exert a force on the cytoskeleton during this cycle. The release of energy by ATP hydrolysis powers
this cycling process. After the action potential ends, Ca 2+ is removed by active transport into the
sarcoplasmic reticulum. The tropomyosin is then restored to block the myosin-binding site, the con-
traction then ends and muscle fiber relaxes.
Sarcolemma
Sarcoplasmic 1
reticulum
Ca 2+ Ca 2+ Transverse
tubule
2
Ca 2+ release and reuptake
Ca 2+
Tropomyosin Troponin
3
Actin
4
Myosin
FIGURE 12.3 The four steps in excitation-contraction coupling (see text for
details). [Adapted and modified from Fitts and Metzger (1993).]