Page 111 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 111
90 MEDICAL DEVICE DESIGN
A B
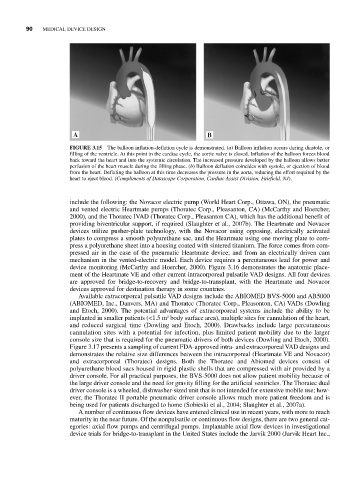
FIGURE 3.15 The balloon inflation-deflation cycle is demonstrated. (a) Balloon inflation occurs during diastole, or
filling of the ventricle. At this point in the cardiac cycle, the aortic valve is closed. Inflation of the balloon forces blood
back toward the heart and into the systemic circulation. The increased pressure developed by the balloon allows better
perfusion of the heart muscle during the filling phase. (b) Balloon deflation coincides with systole, or ejection of blood
from the heart. Deflating the balloon at this time decreases the pressure in the aorta, reducing the effort required by the
heart to eject blood. (Compliments of Datascope Corporation, Cardiac Assist Division, Fairfield, NJ).
include the following: the Novacor electric pump (World Heart Corp., Ottawa, ON), the pneumatic
and vented electric Heartmate pumps (Thoratec Corp., Pleasanton, CA) (McCarthy and Hoercher,
2000), and the Thoratec IVAD (Thoratec Corp., Pleasanton CA), which has the additional benefit of
providing biventricular support, if required (Slaughter et al., 2007b). The Heartmate and Novacor
devices utilize pusher-plate technology, with the Novacor using opposing, electrically activated
plates to compress a smooth polyurethane sac, and the Heartmate using one moving plate to com-
press a polyurethane sheet into a housing coated with sintered titanium. The force comes from com-
pressed air in the case of the pneumatic Heartmate device, and from an electrically driven cam
mechanism in the vented-electric model. Each device requires a percutaneous lead for power and
device monitoring (McCarthy and Hoercher, 2000). Figure 3.16 demonstrates the anatomic place-
ment of the Heartmate VE and other current intracorporeal pulsatile VAD designs. All four devices
are approved for bridge-to-recovery and bridge-to-transplant, with the Heartmate and Novacor
devices approved for destination therapy in some countries.
Available extracorporeal pulsatile VAD designs include the ABIOMED BVS-5000 and AB5000
(ABIOMED, Inc., Danvers, MA) and Thoratec (Thoratec Corp., Pleasonton, CA) VADs (Dowling
and Etoch, 2000). The potential advantages of extracorporeal systems include the ability to be
implanted in smaller patients (<1.5 m body surface area), multiple sites for cannulation of the heart,
2
and reduced surgical time (Dowling and Etoch, 2000). Drawbacks include large percutaneous
cannulation sites with a potential for infection, plus limited patient mobility due to the larger
console size that is required for the pneumatic drivers of both devices (Dowling and Etoch, 2000).
Figure 3.17 presents a sampling of current FDA-approved intra- and extracorporeal VAD designs and
demonstrates the relative size differences between the intracorporeal (Heartmate VE and Novacor)
and extracorporeal (Thoratec) designs. Both the Thoratec and Abiomed devices consist of
polyurethane blood sacs housed in rigid plastic shells that are compressed with air provided by a
driver console. For all practical purposes, the BVS-5000 does not allow patient mobility because of
the large driver console and the need for gravity filling for the artificial ventricles. The Thoratec dual
driver console is a wheeled, dishwasher-sized unit that is not intended for extensive mobile use; how-
ever, the Thoratec II portable pneumatic driver console allows much more patient freedom and is
being used for patients discharged to home (Sobieski et al., 2004; Slaughter et al., 2007a).
A number of continuous flow devices have entered clinical use in recent years, with more to reach
maturity in the near future. Of the nonpulsatile or continuous flow designs, there are two general cat-
egories: axial flow pumps and centrifugal pumps. Implantable axial flow devices in investigational
device trials for bridge-to-transplant in the United States include the Jarvik 2000 (Jarvik Heart Inc.,