Page 114 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 114
OVERVIEW OF CARDIOVASCULAR DEVICES 93
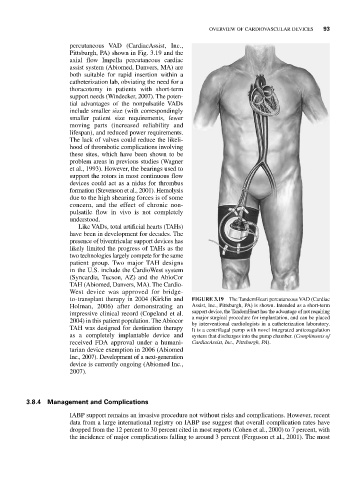
percutaneous VAD (CardiacAssist, Inc.,
Pittsburgh, PA) shown in Fig. 3.19 and the
axial flow Impella percutaneous cardiac
assist system (Abiomed, Danvers, MA) are
both suitable for rapid insertion within a
catheterization lab, obviating the need for a
thoracotomy in patients with short-term
support needs (Windecker, 2007). The poten-
tial advantages of the nonpulsatile VADs
include smaller size (with correspondingly
smaller patient size requirements, fewer
moving parts (increased reliability and
lifespan), and reduced power requirements.
The lack of valves could reduce the likeli-
hood of thrombotic complications involving
these sites, which have been shown to be
problem areas in previous studies (Wagner
et al., 1993). However, the bearings used to
support the rotors in most continuous flow
devices could act as a nidus for thrombus
formation (Stevenson et al., 2001). Hemolysis
due to the high shearing forces is of some
concern, and the effect of chronic non-
pulsatile flow in vivo is not completely
understood.
Like VADs, total artificial hearts (TAHs)
have been in development for decades. The
presence of biventricular support devices has
likely limited the progress of TAHs as the
two technologies largely compete for the same
patient group. Two major TAH designs
in the U.S. include the CardioWest system
(Syncardia, Tucson, AZ) and the AbioCor
TAH (Abiomed, Danvers, MA). The Cardio-
West device was approved for bridge-
to-transplant therapy in 2004 (Kirklin and FIGURE 3.19 The TandemHeart percutaneous VAD (Cardiac
Holman, 2006) after demonstrating an Assist, Inc., Pittsburgh, PA) is shown. Intended as a short-term
impressive clinical record (Copeland et al. support device, the TandemHeart has the advantage of not requiring
a major surgical procedure for implantation, and can be placed
2004) in this patient population. The Abiocor by interventional cardiologists in a catheterization laboratory.
TAH was designed for destination therapy It is a centrifugal pump with novel integrated anticoagulation
as a completely implantable device and system that discharges into the pump chamber. (Compliments of
received FDA approval under a humani- CardiacAssist, Inc., Pittsburgh, PA).
tarian device exemption in 2006 (Abiomed
Inc., 2007). Development of a next-generation
device is currently ongoing (Abiomed Inc.,
2007).
3.8.4 Management and Complications
IABP support remains an invasive procedure not without risks and complications. However, recent
data from a large international registry on IABP use suggest that overall complication rates have
dropped from the 12 percent to 30 percent cited in most reports (Cohen et al., 2000) to 7 percent, with
the incidence of major complications falling to around 3 percent (Ferguson et al., 2001). The most