Page 113 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 113
92 MEDICAL DEVICE DESIGN
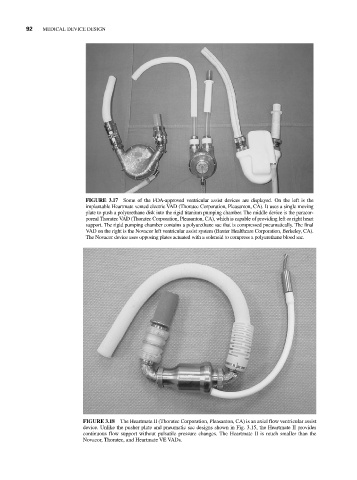
FIGURE 3.17 Some of the FDA-approved ventricular assist devices are displayed. On the left is the
implantable Heartmate vented electric VAD (Thoratec Corporation, Pleasanton, CA). It uses a single moving
plate to push a polyurethane disk into the rigid titanium pumping chamber. The middle device is the paracor-
poreal Thoratec VAD (Thoratec Corporation, Pleasanton, CA), which is capable of providing left or right heart
support. The rigid pumping chamber contains a polyurethane sac that is compressed pneumatically. The final
VAD on the right is the Novacor left ventricular assist system (Baxter Healthcare Corporation, Berkeley, CA).
The Novacor device uses opposing plates actuated with a solenoid to compress a polyurethane blood sac.
FIGURE 3.18 The Heartmate II (Thoratec Corporation, Pleasanton, CA) is an axial flow ventricular assist
device. Unlike the pusher plate and pneumatic sac designs shown in Fig. 3.15, the Heartmate II provides
continuous flow support without pulsatile pressure changes. The Heartmate II is much smaller than the
Novacor, Thoratec, and Heartmate VE VADs.