Page 231 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 231
210 MEDICAL DEVICE DESIGN
Production
packages
Bioburden
nominal sealing
parameters
Distribution
STERILIZATION Product
simulation
(w/validated process) sterility test
ASTM D 4169
Package integrity
Seal strength tests Seal strength tests
tests (Leak)
Pkgs meet
NO spec.
YES
PQ Completed
DOCUMENT
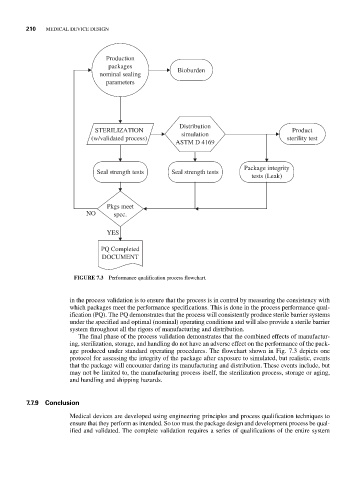
FIGURE 7.3 Performance qualification process flowchart.
in the process validation is to ensure that the process is in control by measuring the consistency with
which packages meet the performance specifications. This is done in the process performance qual-
ification (PQ). The PQ demonstrates that the process will consistently produce sterile barrier systems
under the specified and optimal (nominal) operating conditions and will also provide a sterile barrier
system throughout all the rigors of manufacturing and distribution.
The final phase of the process validation demonstrates that the combined effects of manufactur-
ing, sterilization, storage, and handling do not have an adverse effect on the performance of the pack-
age produced under standard operating procedures. The flowchart shown in Fig. 7.3 depicts one
protocol for assessing the integrity of the package after exposure to simulated, but realistic, events
that the package will encounter during its manufacturing and distribution. These events include, but
may not be limited to, the manufacturing process itself, the sterilization process, storage or aging,
and handling and shipping hazards.
7.7.9 Conclusion
Medical devices are developed using engineering principles and process qualification techniques to
ensure that they perform as intended. So too must the package design and development process be qual-
ified and validated. The complete validation requires a series of qualifications of the entire system