Page 236 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 236
STERILE MEDICAL DEVICE PACKAGE DEVELOPMENT 215
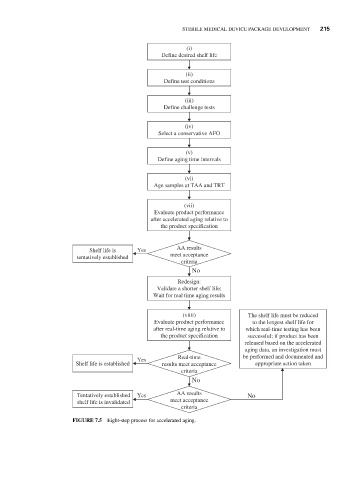
(i)
Define desired shelf life
(ii)
Define test conditions
(iii)
Define challenge tests
(iv)
Select a conservative AFO
(v)
Define aging time intervals
(vi)
Age samples at TAA and TRT
(vii)
Evaluate product performance
after accelerated aging relative to
the product specification
Shelf life is Yes AA results
tentatively established meet acceptance
criteria
No
Redesign:
Validate a shorter shelf life;
Wait for real time aging results
(viiii) The shelf life must be reduced
Evaluate product performance to the longest shelf life for
after real-time aging relative to which real-time testing has been
the product specification successful; if product has been
released based on the accelerated
aging data, an investigation must
Real-time be performed and documented and
Yes
Shelf life is established results meet acceptance appropriate action taken
criteria
No
AA results
Tentatively established Yes No
shelf life is invalidated meet acceptance
criteria
FIGURE 7.5 Eight-step process for accelerated aging.