Page 237 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 237
216 MEDICAL DEVICE DESIGN
Concentration of water in air as a function of temperature
and relative humidity
100% Rh
90% Rh
140000
80% Rh
70% Rh
120000
60% Rh
50% Rh
Concentration (ppm) 80000 30% Rh
100000
40% Rh
20% Rh
60000
10% Rh
40000
20000
0
20 30 40 50 60
Dry bulb T (°C)
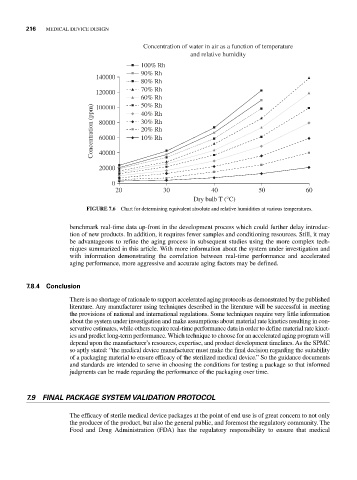
FIGURE 7.6 Chart for determining equivalent absolute and relative humidities at various temperatures.
benchmark real-time data up-front in the development process which could further delay introduc-
tion of new products. In addition, it requires fewer samples and conditioning resources. Still, it may
be advantageous to refine the aging process in subsequent studies using the more complex tech-
niques summarized in this article. With more information about the system under investigation and
with information demonstrating the correlation between real-time performance and accelerated
aging performance, more aggressive and accurate aging factors may be defined.
7.8.4 Conclusion
There is no shortage of rationale to support accelerated aging protocols as demonstrated by the published
literature. Any manufacturer using techniques described in the literature will be successful in meeting
the provisions of national and international regulations. Some techniques require very little information
about the system under investigation and make assumptions about material rate kinetics resulting in con-
servative estimates, while others require real-time performance data in order to define material rate kinet-
ics and predict long-term performance. Which technique to choose for an accelerated aging program will
depend upon the manufacturer’s resources, expertise, and product development timelines. As the SPMC
so aptly stated: “the medical device manufacturer must make the final decision regarding the suitability
of a packaging material to ensure efficacy of the sterilized medical device.” So the guidance documents
and standards are intended to serve in choosing the conditions for testing a package so that informed
judgments can be made regarding the performance of the packaging over time.
7.9 FINAL PACKAGE SYSTEM VALIDATION PROTOCOL
The efficacy of sterile medical device packages at the point of end use is of great concern to not only
the producer of the product, but also the general public, and foremost the regulatory community. The
Food and Drug Administration (FDA) has the regulatory responsibility to ensure that medical