Page 234 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 234
STERILE MEDICAL DEVICE PACKAGE DEVELOPMENT 213
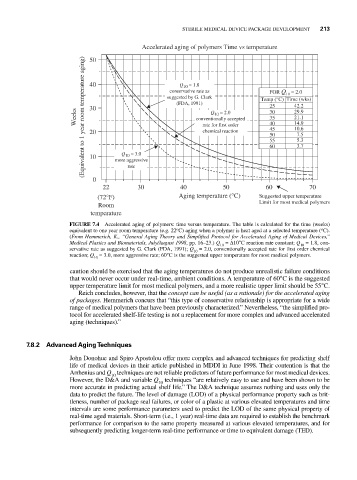
Accelerated aging of polymers Time vs temperature
(Equivalent to 1 year room temperature aging) 20 conventionally accepted 35 14.9
50
Q = 1.8
10
conservative rate as
FOR Q = 2.0
Time (wks)
(FDA, 1991)
25
30
Weeks 40 suggested by G. Clark Q = 2.0 Temp (°C) 10 42.2
29.9
30
10
21.1
40
rate for first order
10.6
45
chemical reaction
50
7.5
5.3
55
3.7
60
Q = 3.0
10
10
rate
0 more aggressive
22 30 40 50 60 70
(72°F) Aging temperature (°C) Suggested upper temperature
Limit for most medical polymers
Room
temperature
FIGURE 7.4 Accelerated aging of polymers: time versus temperature. The table is calculated for the time (weeks)
ο
ο
equivalent to one year room temperature (e.g. 22 C) aging when a polymer is heat aged at a selected temperature ( C).
(From Hemmerich, K., “General Aging Theory and Simplified Protocol for Accelerated Aging of Medical Devices,”
Medical Plastics and Biomaterials, July/August 1998, pp. 16–23.) Q = Δ10°C reaction rate constant; Q = 1.8, con-
10 10
servative rate as suggested by G. Clark (FDA, 1991); Q = 2.0, conventionally accepted rate for first order chemical
10
reaction; Q = 3.0, more aggressive rate; 60°C is the suggested upper temperature for most medical polymers.
10
caution should be exercised that the aging temperatures do not produce unrealistic failure conditions
that would never occur under real-time, ambient conditions. A temperature of 60°C is the suggested
upper temperature limit for most medical polymers, and a more realistic upper limit should be 55°C.
Reich concludes, however, that the concept can be useful (as a rationale) for the accelerated aging
of packages. Hemmerich concurs that “this type of conservative relationship is appropriate for a wide
range of medical polymers that have been previously characterized.” Nevertheless, “the simplified pro-
tocol for accelerated shelf-life testing is not a replacement for more complex and advanced accelerated
aging (techniques).”
7.8.2 Advanced Aging Techniques
John Donohue and Spiro Apostolou offer more complex and advanced techniques for predicting shelf
life of medical devices in their article published in MDDI in June 1998. Their contention is that the
Arrhenius and Q techniques are not reliable predictors of future performance for most medical devices.
10
However, the D&A and variable Q techniques “are relatively easy to use and have been shown to be
10
more accurate in predicting actual shelf life.” The D&A technique assumes nothing and uses only the
data to predict the future. The level of damage (LOD) of a physical performance property such as brit-
tleness, number of package seal failures, or color of a plastic at various elevated temperatures and time
intervals are some performance parameters used to predict the LOD of the same physical property of
real-time aged materials. Short-term (i.e., 1 year) real-time data are required to establish the benchmark
performance for comparison to the same property measured at various elevated temperatures, and for
subsequently predicting longer-term real-time performance or time to equivalent damage (TED).