Page 239 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 239
218 MEDICAL DEVICE DESIGN
Single sterile barrier
Systems Conceptual pkg. validation
for
(Produced at worst-case
sterile barrier system
process limits)
Sterile barrier
Baseline/ systems
control
Without product
Seal strength test
Sterilization
EtO Sterile barrier
systems
Seal integrity test
with product
Sterile barrier
systems
Without product
Seal strength test
Seal strength test 3 Year AA Environmental
ASTM F1980 extremes
Accel. Aged
55C/10% Seal integrity test
Seal integrity test 114 days
Seal strength test
Distribution
simulation
Seal integrity test
Final test report
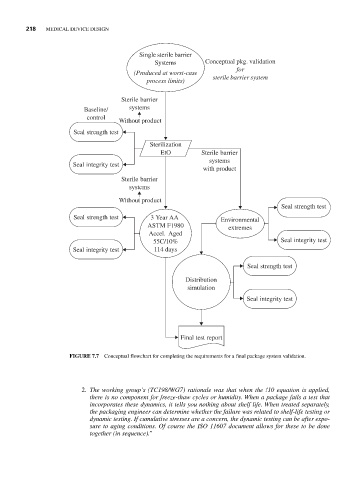
FIGURE 7.7 Conceptual flowchart for completing the requirements for a final package system validation.
2. The working group’s (TC198/WG7) rationale was that when the !10 equation is applied,
there is no component for freeze-thaw cycles or humidity. When a package fails a test that
incorporates these dynamics, it tells you nothing about shelf life. When treated separately,
the packaging engineer can determine whether the failure was related to shelf-life testing or
dynamic testing. If cumulative stresses are a concern, the dynamic testing can be after expo-
sure to aging conditions. Of course the ISO 11607 document allows for these to be done
together (in sequence).”