Page 253 - Carbon Nanotube Fibres and Yarns
P. 253
244 Carbon Nanotube Fibers and Yarns
10 7
10 6 5 Capacitors
Specific power (W kg –1 ) 10 4 3 Electrochemical
10
10
capacitors
2
10
Batteries Fuel
10 cells
0.01 0.1 1 10 100 1000
–1
Specific energy (Wh kg )
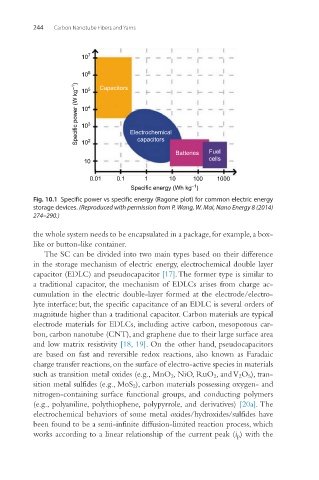
Fig. 10.1 Specific power vs specific energy (Ragone plot) for common electric energy
storage devices. (Reproduced with permission from P. Wang, W. Mai, Nano Energy 8 (2014)
274–290.)
the whole system needs to be encapsulated in a package, for example, a box-
like or button-like container.
The SC can be divided into two main types based on their difference
in the storage mechanism of electric energy, electrochemical double layer
capacitor (EDLC) and pseudocapacitor [17]. The former type is similar to
a traditional capacitor, the mechanism of EDLCs arises from charge ac-
cumulation in the electric double-layer formed at the electrode/electro-
lyte interface; but, the specific capacitance of an EDLC is several orders of
magnitude higher than a traditional capacitor. Carbon materials are typical
electrode materials for EDLCs, including active carbon, mesoporous car-
bon, carbon nanotube (CNT), and graphene due to their large surface area
and low matrix resistivity [18, 19]. On the other hand, pseudocapacitors
are based on fast and reversible redox reactions, also known as Faradaic
charge transfer reactions, on the surface of electro-active species in materials
such as transition metal oxides (e.g., MnO 2 , NiO, RuO 2 , and V 2 O 5 ), tran-
sition metal sulfides (e.g., MoS 2 ), carbon materials possessing oxygen- and
nitrogen-containing surface functional groups, and conducting polymers
(e.g., polyaniline, polythiophene, polypyrrole, and derivatives) [20a]. The
electrochemical behaviors of some metal oxides/hydroxides/sulfides have
been found to be a semi-infinite diffusion-limited reaction process, which
works according to a linear relationship of the current peak (i p ) with the