Page 84 - Carbon Nanotubes
P. 84
Topological and sp3 defect structures in nanotubes 13
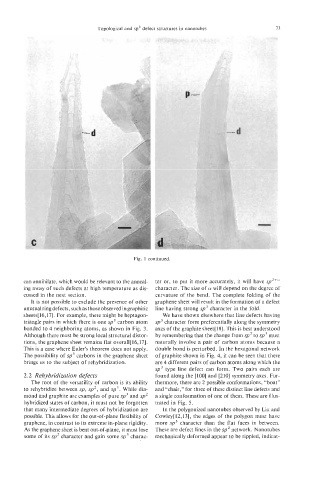
Fig. 1 continued.
can annihilate, which would be relevant to the anneal- ter or, to put it more accurately, it will have spZfLV
ing away of such defects at high temperature as dis- character. The size of CY will depend on the degree of
cussed in the next section. curvature of the bend. The complete folding of the
It is not possible to exclude the presence of other graphene sheet will result in the formation of a defect
unusual ring defects, such as those observed in graphitic line having strong sp3 character in the fold.
sheets[ 16,171. For example, there might be heptagon- We have shown elsewhere that line defects having
triangle pairs in which there is one sp3 carbon atom sp3 character form preferentially along the symmetry
bonded to 4 neighboring atoms, as shown in Fig. 3. axes of the graphite sheet[lb]. This is best understood
Although there must be strong local structural distor- by remembering that the change from sp2 to sp3 must
tions, the graphene sheet remains flat overa11[16,17]. naturally involve a pair of carbon atoms because a
This is a case where Euler’s theorem does not apply. double bond is perturbed. In the hexagonal network
The possibility of sp3 carbons in the graphene sheet of graphite shown in Fig. 4, it can be seen that there
brings us to the subject of rehybridization. are 4 different pairs of carbon atoms along which the
sp3 type line defect can form. Two pairs each are
2.2 Rehybridization defects found along the [loo] and [210] symmetry axes. Fur-
The root of the versatility of carbon is its ability thermore, there are 2 possible conformations, “boat”
to rehybridize between sp, sp2, and sp3. While dia- and “chair,” for three of these distinct line defects and
mond and graphite are examples of pure sp3 and sp2 a single conformation of one of them. These are illus-
hybridized states of carbon, it must not be forgotten trated in Fig. 5.
that many intermediate degrees of hybridization are In the polygonized nanotubes observed by Liu and
possible. This allows for the out-of-plane flexibility of Cowley[12,13], the edges of the polygon must have
graphene, in contrast to its extreme in-plane rigidity. more sp3 character than the flat faces in between.
As the graphene sheet is bent out-of-plane, it must lose These are defect lines in the sp2 network. Nanotubes
some of its sp2 character and gain some sp3 charac- mechanically deformed appear to be rippled, indicat-