Page 85 - Carbon Nanotubes
P. 85
74 T. W. EBBESEN and T. TAKADA
/
/ /
/ / /
/
, .
/ /
/ /
/
/
/
/
/
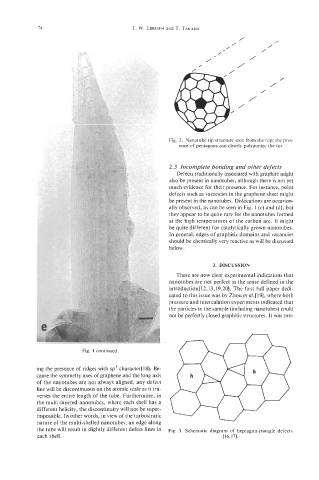
Fig. 2. Nanotube tip structure seen from the top; the pres-
ence of pentagons can clearly polygonize the tip.
2.3 Incomplete bonding and other defects
Defects traditionally associated with graphite might
also be present in nanotubes, although there is not yet
much evidence for their presence. For instance, point
defects such as vacancies in the graphene sheet might
be present in the nanotubes. Dislocations are occasion-
ally observed, as can be seen in Fig. 1 (c) and (d), but
they appear to be quite rare for the nanotubes formed
at the high temperatures of the carbon arc. It might
be quite different for catalytically grown nanotubes.
In general, edges of graphitic domains and vacancies
should be chemically very reactive as will be discussed
below.
3. DISCUSSION
There are now clear experimental indications that
nanotubes are not perfect in the sense defined in the
introduction[l2,13,19,20]. The first full paper dedi-
cated to this issue was by Zhou et al.[19], where both
pressure and intercalation experiments indicated that
the particles in the sample (including nanotubes) could
not be perfectly closed graphitic structures. It was pro-
I
Fig. 1 continued.
ing the presence of ridges with sp3 character[l8]. Be-
cause the symmetry axes of graphene and the long axis
of the nanotubes are not always aligned, any defect
line will be discontinuous on the atomic scale as it tra-
verses the entire length of the tube. Furthermore, in
the multi-layered nanotubes, where each shell has a
different helicity, the discontinuity will not be super-
imposable. In other words, in view of the turbostratic
nature of the multi-shelled nanotubes, an edge along
the tube will result in slightly different defect lines in Fig. 3. Schematic diagram of heptagon-triangle defects
each shell. [ 16,171.