Page 16 - Carbonate Sedimentology and Sequence Stratigraphy
P. 16
CHAPTER 1: ESSENTIALS OF NEIGHBORING DISCIPLINES 7
50°
0°
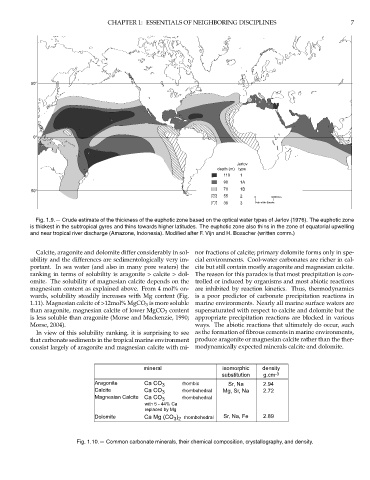
Jerlov
depth (m) type
110 1
90 1A
70 1B
50°
55 2 0 3000 Km
30 3 Scale at the Equator.
Fig. 1.9.— Crude estimate of the thickness of the euphotic zone based on the optical water types of Jerlov (1976). The euphotic zone
is thickest in the subtropical gyres and thins towards higher latitudes. The euphotic zone also thins in the zone of equatorial upwelling
and near tropical river discharge (Amazone, Indonesia). Modified after F. Vijn and H. Bosscher (written comm.)
Calcite, aragonite and dolomite differ considerably in sol- nor fractions of calcite; primary dolomite forms only in spe-
ubility and the differences are sedimentologically very im- cial environments. Cool-water carbonates are richer in cal-
portant. In sea water (and also in many pore waters) the cite but still contain mostly aragonite and magnesian calcite.
ranking in terms of solubility is aragonite > calcite > dol- The reason for this paradox is that most precipitation is con-
omite. The solubility of magnesian calcite depends on the trolled or induced by organisms and most abiotic reactions
magnesium content as explained above. From 4 mol% on- are inhibited by reaction kinetics. Thus, thermodynamics
wards, solubility steadily increases with Mg content (Fig. is a poor predictor of carbonate precipitation reactions in
1.11). Magnesian calcite of >12mol% MgCO 3 is more soluble marine environments. Nearly all marine surface waters are
than aragonite, magnesian calcite of lower MgCO 3 content supersaturated with respect to calcite and dolomite but the
is less soluble than aragonite (Morse and Mackenzie, 1990; appropriate precipitation reactions are blocked in various
Morse, 2004). ways. The abiotic reactions that ultimately do occur, such
In view of this solubility ranking, it is surprising to see as the formation of fibrous cements in marine environments,
that carbonate sediments in the tropical marine environment produce aragonite or magnesian calcite rather than the ther-
consist largely of aragonite and magnesian calcite with mi- modynamically expected minerals calcite and dolomite.
mineral isomorphic density
substitution g.cm -3
Aragonite Ca CO rhombic Sr, Na 2.94
3
Calcite Ca CO rhombohedral Mg, Sr, Na 2.72
3
Magnesian Calcite Ca CO rhombohedral
3
with 5 - 44% Ca
replaced by Mg
Dolomite Ca Mg (CO ) rhombohedral Sr, Na, Fe 2.89
3 2
Fig. 1.10.— Common carbonate minerals, their chemical composition, crystallography, and density.