Page 17 - Carbonate Sedimentology and Sequence Stratigraphy
P. 17
8 WOLFGANG SCHLAGER
keep up
calcite magnesian calcite
7.0 rapid
creation of
niche
7.2
catch up
7.2
7.2 rate of growth start up
log IAP magnesian calcite 8.2 bonate production. Populations of organisms respond to the open-
7.2
time
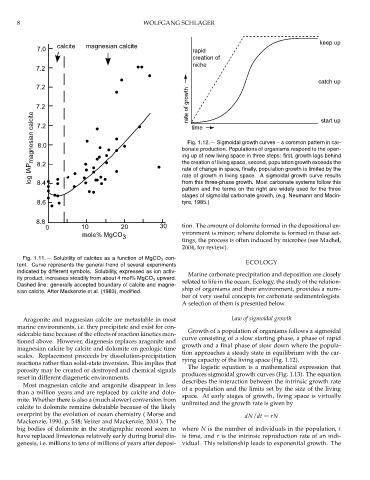
Fig. 1.12.— Sigmoidal growth curves – a common pattern in car-
8.0
ing up of new living space in three steps: first, growth lags behind
the creation of living space, second, population growth exceeds the
rate of change in space, finally, population growth is limited by the
rate of growth in living space. A sigmoidal growth curve results
from this three-phase growth. Most carbonate systems follow this
8.4
pattern and the terms on the right are widely used for the three
stages of sigmoidal carbonate growth, (e.g. Neumann and Macin-
8.6 tyre, 1985.)
8.8
0 10 20 30 tion. The amount of dolomite formed in the depositional en-
mole% MgCO 3 vironment is minor; where dolomite is formed in these set-
tings, the process is often induced by microbes (see Machel,
2004, for review).
Fig. 1.11.— Solubility of calcites as a function of MgCO 3 con-
tent. Curve represents the general trend of several experiments ECOLOGY
indicated by different symbols. Solubility, expressed as ion activ- Marine carbonate precipitation and deposition are closely
ity product, increases steadily from about 4 mol% MgCO 3 upward.
Dashed line: generally accepted boundary of calcite and magne- related to life in the ocean. Ecology, the study of the relation-
sian calcite. After Mackenzie et al. (1983), modified. ship of organisms and their environment, provides a num-
ber of very useful concepts for carbonate sedimentologists.
A selection of them is presented below.
Aragonite and magnesian calcite are metastable in most Law of sigmoidal growth
marine environments, i.e. they precipitate and exist for con-
Growth of a population of organisms follows a sigmoidal
siderable time because of the effects of reaction kinetics men-
curve consisting of a slow starting phase, a phase of rapid
tioned above. However, diagenesis replaces aragonite and
growth and a final phase of slow down where the popula-
magnesian calcite by calcite and dolomite on geologic time
tion approaches a steady state in equilibrium with the car-
scales. Replacement proceeds by dissolution-precipitation
rying capacity of the living space (Fig. 1.12).
reactions rather than solid-state inversion. This implies that The logistic equation is a mathematical expression that
porosity may be created or destroyed and chemical signals produces sigmoidal growth curves (Fig. 1.13). The equation
reset in different diagenetic environments. describes the interaction between the intrinsic growth rate
Most magnesian calcite and aragonite disappear in less
than a million years and are replaced by calcite and dolo- of a population and the limits set by the size of the living
space. At early stages of growth, living space is virtually
mite. Whether there is also a (much slower) conversion from unlimited and the growth rate is given by
calcite to dolomite remains debatable because of the likely
overprint by the evolution of ocean chemistry ( Morse and dN/dt = rN
Mackenzie, 1990, p. 548; Veizer and Mackenzie, 2004 ). The
big bodies of dolomite in the stratigraphic record seem to where N is the number of individuals in the population, t
have replaced limestones relatively early during burial dia- is time, and r is the intrinsic reproduction rate of an indi-
genesis, i.e. millions to tens of millions of years after deposi- vidual. This relationship leads to exponential growth. The