Page 373 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 373
336 Carraher’s Polymer Chemistry
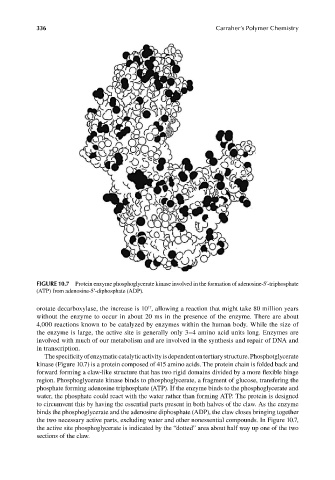
FIGURE 10.7 Protein enzyme phosphoglycerate kinase involved in the formation of adenosine-5′-triphosphate
(ATP) from adenosine-5’-diphosphate (ADP).
orotate decarboxylase, the increase is 10 , allowing a reaction that might take 80 million years
17
without the enzyme to occur in about 20 ms in the presence of the enzyme. There are about
4,000 reactions known to be catalyzed by enzymes within the human body. While the size of
the enzyme is large, the active site is generally only 3–4 amino acid units long. Enzymes are
involved with much of our metabolism and are involved in the synthesis and repair of DNA and
in transcription.
The specificity of enzymatic catalytic activity is dependent on tertiary structure. Phosphotglycerate
kinase (Figure 10.7) is a protein composed of 415 amino acids. The protein chain is folded back and
forward forming a claw-like structure that has two rigid domains divided by a more fl exible hinge
region. Phosphoglycerate kinase binds to phosphoglycerate, a fragment of glucose, transfering the
phosphate forming adenosine triphosphate (ATP). If the enzyme binds to the phosphoglycerate and
water, the phosphate could react with the water rather than forming ATP. The protein is designed
to circumvent this by having the essential parts present in both halves of the claw. As the enzyme
binds the phosphoglycerate and the adenosine diphosphate (ADP), the claw closes bringing together
the two necessary active parts, excluding water and other nonessential compounds. In Figure 10.7,
the active site phosphoglycerate is indicated by the “dotted” area about half way up one of the two
sections of the claw.
9/14/2010 3:41:13 PM
K10478.indb 336 9/14/2010 3:41:13 PM
K10478.indb 336