Page 374 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 374
Naturally Occurring Polymers—Animals 337
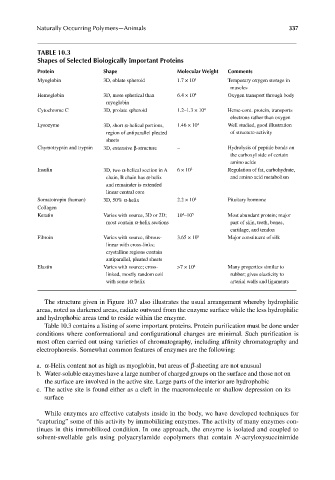
TABLE 10.3
Shapes of Selected Biologically Important Proteins
Protein Shape Molecular Weight Comments
Myoglobin 3D, oblate spheroid 1.7 × 10 4 Temporary oxygen storage in
muscles
Hemoglobin 3D, more spherical than 6.4 × 10 4 Oxygen transport through body
myoglobin
Cytochrome C 3D, prolate spheroid 1.2–1.3 × 10 4 Heme-cont. protein, transports
electrons rather than oxygen
Lysozyme 3D, short α-helical portions, 1.46 × 10 4 Well studied, good illustration
region of antiparallel pleated of structure-activity
sheets
Chymotrypsin and trypsin 3D, extensive β-structure – Hydrolysis of peptide bonds on
the carboxyl side of certain
amino acids
Insulin 3D, two α-helical section in A 6 × 10 3 Regulation of fat, carbohydrate,
chain, B chain has α-helix and amino acid metabolism
and remainder is extended
linear central core
Somatotropin (human) 3D, 50% α-helix 2.2 × 10 4 Pituitary hormone
Collagen
Keratin Varies with source, 3D or 2D; 10 –10 5 Most abundant protein; major
4
most contain α-helix sections part of skin, teeth, bones,
cartilage, and tendon
5
Fibroin Varies with source, fi brous- 3.65 × 10 Major constituent of silk
linear with cross-links;
crystalline regions contain
antiparallel, pleated sheets
Elastin Varies with source; cross- >7 × 10 4 Many properties similar to
linked, mostly random coil rubber; gives elasticity to
with some α-helix arterial walls and ligaments
The structure given in Figure 10.7 also illustrates the usual arrangement whereby hydrophilic
areas, noted as darkened areas, radiate outward from the enzyme surface while the less hydrophilic
and hydrophobic areas tend to reside within the enzyme.
Table 10.3 contains a listing of some important proteins. Protein purification must be done under
conditions where conformational and configurational changes are minimal. Such purifi cation is
most often carried out using varieties of chromatography, including affinity chromatography and
electrophoresis. Somewhat common features of enzymes are the following:
a. α-Helix content not as high as myoglobin, but areas of β-sheeting are not unusual
b. Water-soluble enzymes have a large number of charged groups on the surface and those not on
the surface are involved in the active site. Large parts of the interior are hydrophobic
c. The active site is found either as a cleft in the macromolecule or shallow depression on its
surface
While enzymes are effective catalysts inside in the body, we have developed techniques for
“capturing” some of this activity by immobilizing enzymes. The activity of many enzymes con-
tinues in this immobilized condition. In one approach, the enzyme is isolated and coupled to
solvent-swellable gels using polyacrylamide copolymers that contain N-acryloxysuccinimide
9/14/2010 3:41:14 PM
K10478.indb 337 9/14/2010 3:41:14 PM
K10478.indb 337