Page 377 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 377
340 Carraher’s Polymer Chemistry
CH 3
NH
O
H
H
H N N N N N
H R O R
N O N H
N N
H
N
N N N NH
R R
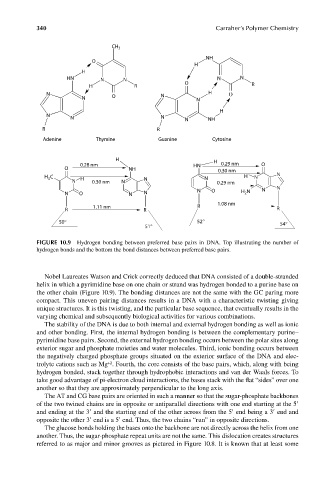
Adenine Thymine Guanine Cytosine
H H
0.28 nm H N 0.29 nm O
O NH 0.30 nm
H 3 C H N N H N N
N 0.30 nm N 0.29 nm
2
N O N N N O H N N N
1.08 nm
1.11 nm R
R R R
50° 52° 54°
51°
FIGURE 10.9 Hydrogen bonding between preferred base pairs in DNA. Top illustrating the number of
hydrogen bonds and the bottom the bond distances between preferred base pairs.
Nobel Laureates Watson and Crick correctly deduced that DNA consisted of a double-stranded
helix in which a pyrimidine base on one chain or strand was hydrogen bonded to a purine base on
the other chain (Figure 10.9). The bonding distances are not the same with the GC paring more
compact. This uneven pairing distances results in a DNA with a characteristic twisting giving
unique structures. It is this twisting, and the particular base sequence, that eventually results in the
varying chemical and subsequently biological activities for various combinations.
The stability of the DNA is due to both internal and external hydrogen bonding as well as ionic
and other bonding. First, the internal hydrogen bonding is between the complementary purine–
pyrimidine base pairs. Second, the external hydrogen bonding occurs between the polar sites along
exterior sugar and phosphate moieties and water molecules. Third, ionic bonding occurs between
the negatively charged phosphate groups situated on the exterior surface of the DNA and elec-
+2
trolyte cations such as Mg . Fourth, the core consists of the base pairs, which, along with being
hydrogen bonded, stack together through hydrophobic interactions and van der Waals forces. To
take good advantage of pi-electron cloud interactions, the bases stack with the flat “sides” over one
another so that they are approximately perpendicular to the long axis.
The AT and CG base pairs are oriented in such a manner so that the sugar-phosphate backbones
of the two twined chains are in opposite or antiparallel directions with one end starting at the 5′
and ending at the 3′ and the starting end of the other across from the 5′ end being a 3′ end and
opposite the other 3′ end is a 5′ end. Thus, the two chains “run” in opposite directions.
The glucose bonds holding the bases onto the backbone are not directly across the helix from one
another. Thus, the sugar-phosphate repeat units are not the same. This dislocation creates structures
referred to as major and minor grooves as pictured in Figure 10.8. It is known that at least some
9/14/2010 3:41:16 PM
K10478.indb 340
K10478.indb 340 9/14/2010 3:41:16 PM