Page 51 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 51
14 Carraher’s Polymer Chemistry
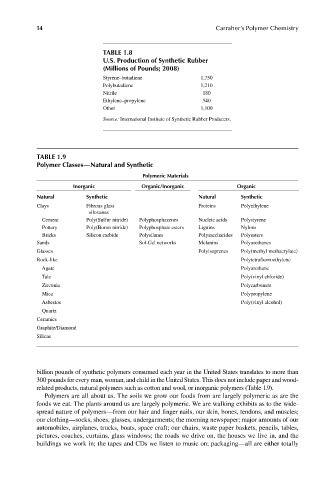
TABLE 1.8
U.S. Production of Synthetic Rubber
(Millions of Pounds; 2008)
Styrene–butadiene 1,750
Polybutadiene 1,210
Nitrile 180
Ethylene–propylene 540
Other 1,100
Source: International Institute of Synthetic Rubber Producers.
TABLE 1.9
Polymer Classes—Natural and Synthetic
Polymeric Materials
Inorganic Organic/Inorganic Organic
Natural Synthetic Natural Synthetic
Clays Fibrous glass Proteins Polyethylene
siloxanes
Cement Poly(Sulfur nitride) Polyphosphazenes Nucleic acids Polystyrene
Pottery Poly(Boron nitride) Polyphosphate esters Lignins Nylons
Bricks Silicon carbide Polysilanes Polysaccharides Polyesters
Sands Sol-Gel networks Melanins Polyurethanes
Glasses Polyisoprenes Poly(methyl methacrylate)
Rock-like Polytetrafl uoroethylene
Agate Polyurethane
Talc Poly(vinyl chloride)
Zirconia Polycarbonate
Mica Polypropylene
Asbestos Poly(vinyl alcohol)
Quartz
Ceramics
Graphite/Diamond
Silicas
billion pounds of synthetic polymers consumed each year in the United States translates to more than
300 pounds for every man, woman, and child in the United States. This does not include paper and wood-
related products, natural polymers such as cotton and wool, or inorganic polymers (Table 1.9).
Polymers are all about us. The soils we grow our foods from are largely polymeric as are the
foods we eat. The plants around us are largely polymeric. We are walking exhibits as to the wide-
spread nature of polymers—from our hair and finger nails, our skin, bones, tendons, and muscles;
our clothing—socks, shoes, glasses, undergarments; the morning newspaper; major amounts of our
automobiles, airplanes, trucks, boats, space craft; our chairs, waste paper baskets, pencils, tables,
pictures, coaches, curtains, glass windows; the roads we drive on, the houses we live in, and the
buildings we work in; the tapes and CDs we listen to music on; packaging—all are either totally
9/14/2010 3:35:52 PM
K10478.indb 14
K10478.indb 14 9/14/2010 3:35:52 PM