Page 651 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 651
614 Carraher’s Polymer Chemistry
A photoresponsive sunglass whose color or tint varies with the intensity of the sunlight is an
example of nonliner optical material. Some of the so-called “smart” windows are also composed
of polymeric materials whose tint varies according to the incident light. Currently much material
is stored using electronic means but optical storage is becoming common place with the use of
CD-ROM and WORM devices. Such storage has the advantages of rapid retrieval and increased
knowledge density (i.e., more information stored in a smaller space).
Since the discovery that doped polyacetylene becomes electrically conductive, a range of poly-
mer-intense semiconductor devices has been studied, including normal transistors and fi eld-effect
transistors (FETs), and photodiodes and light-emitting diodes (LEDs). Like conductive polymers,
these materials obtain their properties because of their electronic nature, specifically the presence
of conjugated pi-bonding systems.
In electrochemical light-emitting cells, the semiconductive polymer can be surrounded asym-
metrically with a hole-injecting material on one side and a low work function electron-injecting
metal (such as magnesium, calcium, or aluminum) on the other side. The emission of light results
from a radiative charge carrier recombining in the polymer as electrons from one side and holes
from the other recombine.
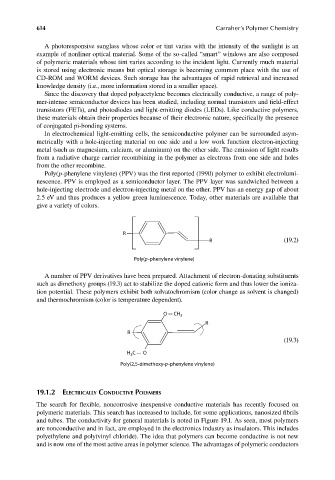
Poly(p-phenylene vinylene) (PPV) was the first reported (1990) polymer to exhibit electrolumi-
nescence. PPV is employed as a semiconductor layer. The PPV layer was sandwiched between a
hole-injecting electrode and electron-injecting metal on the other. PPV has an energy gap of about
2.5 eV and thus produces a yellow green luminescence. Today, other materials are available that
give a variety of colors.
R
R (19.2)
Poly(p-phenylene vinylene)
A number of PPV derivatives have been prepared. Attachment of electron-donating substituents
such as dimethoxy groups (19.3) act to stabilize the doped cationic form and thus lower the ioniza-
tion potential. These polymers exhibit both solvatochromism (color change as solvent is changed)
and thermochromism (color is temperature dependent).
O − CH 3
R
R
(19.3)
H C − O
3
Poly(2,5-dimethoxy-p-phenylene vinylene)
19.1.2 ELECTRICALLY CONDUCTIVE POLYMERS
The search for flexible, noncorrosive inexpensive conductive materials has recently focused on
polymeric materials. This search has increased to include, for some applications, nanosized fi brils
and tubes. The conductivity for general materials is noted in Figure 19.1. As seen, most polymers
are nonconductive and in fact, are employed in the electronics industry as insulators. This includes
polyethylene and poly(vinyl chloride). The idea that polymers can become conductive is not new
and is now one of the most active areas in polymer science. The advantages of polymeric conductors
9/14/2010 3:43:46 PM
K10478.indb 614 9/14/2010 3:43:46 PM
K10478.indb 614