Page 654 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 654
Selected Topics 617
oxidation or reduction resulting in the formation of a bipolaron. The formation of polarons is shown
in Figure 19.2 where resonance structures illustrate the movement of electrons. The bottom reso-
nance structure shows the formation of a bipolaron from the combination of two polarons. While
conductance within a single chain is classically used to illustrate the concept, conductance actually
occurs between chains as well as within chains. Further, for conductance to be effective, transfer-
ence between chains is necessary.
Ordinary polyacetylene is composed of small fi bers (fibrils) that are randomly oriented.
Conductivity is decreased because of the contacts between the various random fi brils. Two
approaches have been taken to align the polyacetylene fibrils. The first approach is to employ a liq-
uid crystal solvent for the acetylene polymerization and to form the polymer under external pertur-
bation. The second approach is to mechanically stretch the polyacetylene material causing the fi brils
to align. The conductivity of polyacetylene is about 100 greater in the direction of the “stretch” in
comparison to that perpendicular to the stretch direction. Thus, the conductivity is isotropic. By
comparison, the conductivity of metals such as copper and silver is anisotropic. Of interest is the
nonconductivity of diamond, which has only ordered sigma-bonds and thus no “movable” electrons
and the isotropic behavior of graphite. Graphite, similar to polyacetylene, has a series of alternating
6
pi bonds (Section 12.16) where the conductivity in the plane of the graphite rings is about 10 times
that at right angles to this plane.
Polyacetylene has been produced by several methods, many utilizing the Zeigler–Natta polymer-
ization systems. Both cis and trans isomers exist (19.4 and 19.5). The cis-polyacetylene is copper
−8
colored with films having a conductivity of about 10 S/m. By comparison, the trans-polyacetylene
−3
is silver colored with films having a much greater conductivity on the order of 10 S/m. The cis iso-
mer is changed into the thermodynamically more stable trans isomer by heating. As noted above,
4
2
conductivity is greatly increased when the trans-polyacetylene is doped (to about 10 –10 S/cm).
Conductivity is dependent on the micro or fine structure of the fibrils, doping agent, extent, and
technique, and aging of the sample.
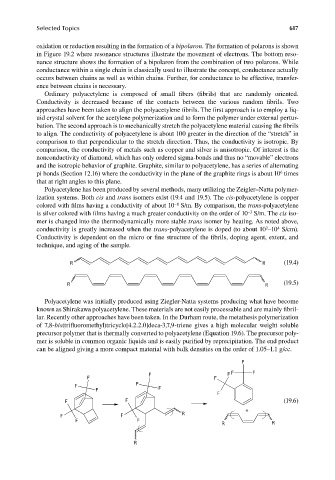
R R (19.4)
R R (19.5)
Polyacetylene was initially produced using Ziegler-Natta systems producing what have become
known as Shirakawa polyacetylene. These materials are not easily processable and are mainly fi bril-
lar. Recently other approaches have been taken. In the Durham route, the metathesis polymerization
of 7,8-bis(trifluoromethyl)tricyclo[4.2.2.0]deca-3,7,9-triene gives a high molecular weight soluble
precursor polymer that is thermally converted to polyacetylene (Equation 19.6). The precursor poly-
mer is soluble in common organic liquids and is easily purified by reprecipitation. The end product
can be aligned giving a more compact material with bulk densities on the order of 1.05–1.1 g/cc.
F
F F F F
F F
F F F
F
F
F F (19.6)
+
F F R
F F
R R
R
9/14/2010 3:43:47 PM
K10478.indb 617 9/14/2010 3:43:47 PM
K10478.indb 617