Page 657 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 657
620 Carraher’s Polymer Chemistry
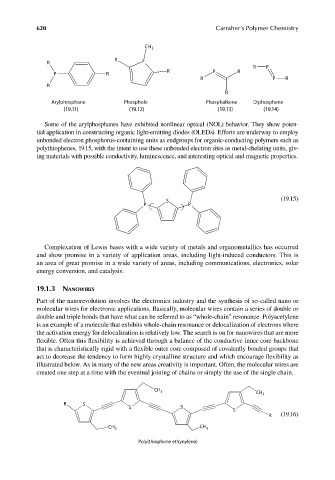
CH 3
R
R P
R P
R P R
P R
R P R
R
R
Arylphosphane Phosphole Phosphalkene Diphosphene
(19.11) (19.12) (19.13) (19.14)
Some of the arylphosphanes have exhibited nonlinear optical (NOL) behavior. They show poten-
tial application in constructing organic light-emitting diodes (OLEDs). Efforts are underway to employ
unbonded electron phosphorus-containing units as endgroups for organic-conducting polymers such as
polythiophenes, 19.15, with the intent to use these unbonded electron sites as metal-chelating units, giv-
ing materials with possible conductivity, luminescence, and interesting optical and magnetic properties.
(19.15)
S
P P
Complexation of Lewis bases with a wide variety of metals and organometallics has occurred
and show promise in a variety of application areas, including light-induced conductors. This is
an area of great promise in a wide variety of areas, including communications, electronics, solar
energy conversion, and catalysis.
19.1.3 NANOWIRES
Part of the nanorevolution involves the electronics industry and the synthesis of so-called nano or
molecular wires for electronic applications. Basically, molecular wires contain a series of double or
double and triple bonds that have what can be referred to as “whole-chain” resonance. Polyacetylene
is an example of a molecule that exhibits whole-chain resonance or delocalization of electrons where
the activation energy for delocalization is relatively low. The search is on for nanowires that are more
flexible. Often this flexibility is achieved through a balance of the conductive inner core backbone
that is characteristically rigid with a flexible outer core composed of covalently bonded groups that
act to decrease the tendency to form highly crystalline structure and which encourage fl exibility as
illustrated below. As in many of the new areas creativity is important. Often, the molecular wires are
created one step at a time with the eventual joining of chains or simply the use of the single chain.
CH 3
CH 3
R S
S S
S
R (19.16)
CH 3 CH 3
Poly(thiophene ethynylene)
9/14/2010 3:43:48 PM
K10478.indb 620 9/14/2010 3:43:48 PM
K10478.indb 620