Page 652 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 652
Selected Topics 615
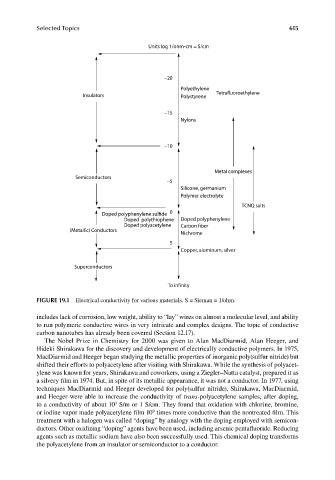
Units log 1/ohm-cm = S/cm
−20
Polyethylene
Tetrafluoroethylene
Insulators Polystyrene
−15
Nylons
−10
Metal complexes
Semiconductors
−5
Silicone, germanium
Polymer electrolyte
TCNQ salts
Doped polyphenylene sulfide 0
Doped polythiophene Doped polyphenylene
Doped polyacetylene Carbon fiber
(Metallic) Conductors
Nichrome
5
Copper, aluminum, silver
Superconductors
To infinity
FIGURE 19.1 Electrical conductivity for various materials. S = Sieman = 1/ohm.
includes lack of corrosion, low weight, ability to “lay” wires on almost a molecular level, and ability
to run polymeric conductive wires in very intricate and complex designs. The topic of conductive
carbon nanotubes has already been covered (Section 12.17).
The Nobel Prize in Chemistry for 2000 was given to Alan MacDiarmid, Alan Heeger, and
Hideki Shirakawa for the discovery and development of electrically conductive polymers. In 1975,
MacDiarmid and Heeger began studying the metallic properties of inorganic poly(sulfur nitride) but
shifted their efforts to polyacetylene after visiting with Shirakawa. While the synthesis of polyacet-
ylene was known for years, Shirakawa and coworkers, using a Ziegler–Natta catalyst, prepared it as
a silvery film in 1974. But, in spite of its metallic appearance, it was not a conductor. In 1977, using
techniques MacDiarmid and Heeger developed for poly(sulfur nitride), Shirakawa, MacDiarmid,
and Heeger were able to increase the conductivity of trans-polyacetylene samples, after doping,
3
to a conductivity of about 10 S/m or 1 S/cm. They found that oxidation with chlorine, bromine,
9
or iodine vapor made polyacetylene fi lm 10 times more conductive than the nontreated fi lm. This
treatment with a halogen was called “doping” by analogy with the doping employed with semicon-
ductors. Other oxidizing “doping” agents have been used, including arsenic pentafl uoride. Reducing
agents such as metallic sodium have also been successfully used. This chemical doping transforms
the polyacetylene from an insulator or semiconductor to a conductor.
9/14/2010 3:43:46 PM
K10478.indb 615 9/14/2010 3:43:46 PM
K10478.indb 615