Page 653 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 653
616 Carraher’s Polymer Chemistry
Doped
C + C C CH +
HC C + C C +
C + C +
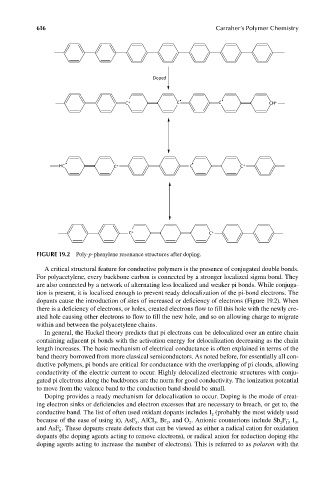
FIGURE 19.2 Poly-p-phenylene resonance structures after doping.
A critical structural feature for conductive polymers is the presence of conjugated double bonds.
For polyacetylene, every backbone carbon is connected by a stronger localized sigma bond. They
are also connected by a network of alternating less localized and weaker pi bonds. While conjuga-
tion is present, it is localized enough to prevent ready delocalization of the pi-bond electrons. The
dopants cause the introduction of sites of increased or deficiency of electrons (Figure 19.2). When
there is a deficiency of electrons, or holes, created electrons flow to fill this hole with the newly cre-
ated hole causing other electrons to fl ow to fill the new hole, and so on allowing charge to migrate
within and between the polyacetylene chains.
In general, the Huckel theory predicts that pi electrons can be delocalized over an entire chain
containing adjacent pi bonds with the activation energy for delocalization decreasing as the chain
length increases. The basic mechanism of electrical conductance is often explained in terms of the
band theory borrowed from more classical semiconductors. As noted before, for essentially all con-
ductive polymers, pi bonds are critical for conductance with the overlapping of pi clouds, allowing
conductivity of the electric current to occur. Highly delocalized electronic structures with conju-
gated pi electrons along the backbones are the norm for good conductivity. The ionization potential
to move from the valence band to the conduction band should be small.
Doping provides a ready mechanism for delocalization to occur. Doping is the mode of creat-
ing electron sinks or deficiencies and electron excesses that are necessary to breach, or get to, the
conductive band. The list of often used oxidant dopants includes I (probably the most widely used
2
−
because of the ease of using it), AsF , AlCl , Br , and O . Anionic counterions include Sb F , I ,
3
2
2
2 11
5
3
and AsF . These dopants create defects that can be viewed as either a radical cation for oxidation
−
6
dopants (the doping agents acting to remove electrons), or radical anion for reduction doping (the
doping agents acting to increase the number of electrons). This is referred to as polaron with the
K10478.indb 616 9/14/2010 3:43:47 PM
9/14/2010 3:43:47 PM
K10478.indb 616