Page 71 - Chalcogenide Glasses for Infrared Optics
P. 71
Chalcogenide Glasses 49
atomic ratios are correct. Now, chemical bonds Ge-S and Sb-S are
thermodynamically favored, from a free energy of formation stand-
point, over S-S bonds. On the sulfur-rich side of that line, there is
more than enough sulfur to satisfy the bonding requirements of both
Ge and Sb. The remaining sulfur is bonded to other sulfur atoms in
chains or rings. However, at the stoichiometric line, all the sulfur is
bonded to either Ge or Sb. There are no longer S-S primary bonds.
Across the line in the metallic rich areas, there is not enough S to go
around. The free energy of formation for Ge-S is greater than that for
Sb-S. Ge will use up its share of S first. But well away from the line we
may expect to find Ge-Sb bonds, Ge-Ge bonds, and Sb-Sb bonds. The
Ge-S bond has a high enough energy level that in binary form it can
transmit visible light. The Ge-Ge, the Ge-Sb, and the Sb-Sb are all
16
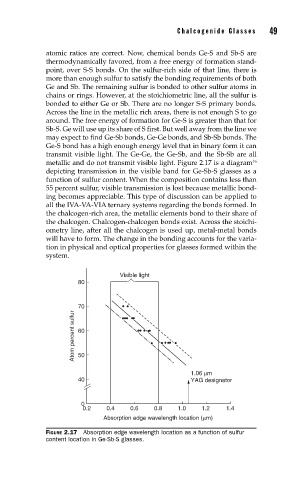
metallic and do not transmit visible light. Figure 2.17 is a diagram
depicting transmission in the visible band for Ge-Sb-S glasses as a
function of sulfur content. When the composition contains less than
55 percent sulfur, visible transmission is lost because metallic bond-
ing becomes appreciable. This type of discussion can be applied to
all the IVA-VA-VIA ternary systems regarding the bonds formed. In
the chalcogen-rich area, the metallic elements bond to their share of
the chalcogen. Chalcogen-chalcogen bonds exist. Across the stoichi-
ometry line, after all the chalcogen is used up, metal-metal bonds
will have to form. The change in the bonding accounts for the varia-
tion in physical and optical properties for glasses formed within the
system.
Visible light
80
70
Atom percent sulfur 60
50
1.06 µm
40 YAG designator
0
0.2 0.4 0.6 0.8 1.0 1.2 1.4
Absorption edge wavelength location (µm)
FIGURE 2.17 Absorption edge wavelength location as a function of sulfur
content location in Ge-Sb-S glasses.