Page 70 - Chalcogenide Glasses for Infrared Optics
P. 70
48 Cha pte r T w o
2.5.7 Softening Points
Softening point decreases with increasing molecular weight.
S > Se > Te
P > As > Sb
Si > Ge > Sn
2.6 Chemical Bonding in Chalcogenide Glasses
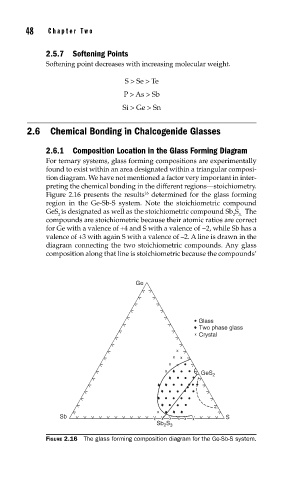
2.6.1 Composition Location in the Glass Forming Diagram
For ternary systems, glass forming compositions are experimentally
found to exist within an area designated within a triangular composi-
tion diagram. We have not mentioned a factor very important in inter-
preting the chemical bonding in the different regions—stoichiometry.
16
Figure 2.16 presents the results determined for the glass forming
region in the Ge-Sb-S system. Note the stoichiometric compound
GeS is designated as well as the stoichiometric compound Sb S The
2 2 3.
compounds are stoichiometric because their atomic ratios are correct
for Ge with a valence of +4 and S with a valence of −2, while Sb has a
valence of +3 with again S with a valence of –2. A line is drawn in the
diagram connecting the two stoichiometric compounds. Any glass
composition along that line is stoichiometric because the compounds’
Ge
Glass
Two phase glass
Crystal
GeS 2
Sb S
Sb 2 S 3
FIGURE 2.16 The glass forming composition diagram for the Ge-Sb-S system.