Page 72 - Chalcogenide Glasses for Infrared Optics
P. 72
50 Cha pte r T w o
2.6.2 Molecular Vibrations of Constituent Atoms
Some insight into the molecular nature of the chalcogenide glasses
may be gained by the use of some standard methods. One method
already mentioned in Chap. 1 is far infrared reflection spectroscopy
to observe the strong Restrahlen like bands due to the constituent
atom pairs. The word like is added because the term is normally used
to describe infrared reflection for crystalline materials, not glasses.
The greater the ionic character of the bond formed between the atom
pair, the more intense the absorption which in turn increases the
magnitude of the reflection. From inspection of the shape of the curve,
37
one can deduce the frequency of the vibration between the two
atoms to a fair degree of accuracy. Of course, if the sample can be
ground thin enough and polished again, the absorption frequency
may be directly measured through transmission. Absorption results
may also be obtained by powdering the material and pressing into a
pellet using KBr or TlBr and measuring IR transmission. Another way,
and the most accurate, is to use a Raman spectrophotometer which
directly measures the frequency of all the intense vibrations. Keep in
mind, such instruments were not readily available at the time of the
results of the program being described.
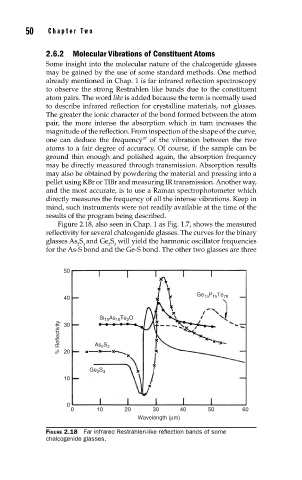
Figure 2.18, also seen in Chap. 1 as Fig. 1.7, shows the measured
reflectivity for several chalcogenide glasses. The curves for the binary
glasses As S and Ge S will yield the harmonic oscillator frequencies
2 3 2 3
for the As-S bond and the Ge-S bond. The other two glasses are three
50
40 Ge 15 P 15 Te 70
Si 10 As 10 Te 2 O
% Reflectivity 20 As 2 S 3
30
Ge 2 S 3
10
0
0 10 20 30 40 50 60
Wavelength (µm)
FIGURE 2.18 Far infrared Restrahlen-like refl ection bands of some
chalcogenide glasses.