Page 77 - Chalcogenide Glasses for Infrared Optics
P. 77
Chalcogenide Glasses 55
occur when compositions move from the chalcogen-rich region
through the stoichiometric line into chalcogen-deficient composi-
tions. No doubt, in this investigation or one similar, Raman spectra
would have been beneficial in trying to untangle observed frequen-
cies as related to structures. Later investigations using Raman results
have been reported. 41
2.6.3 Mass Spectrometric Investigation of Bonding
in the Glasses
Chalcogenide glasses are made from volatile elements. An investiga-
tion using a mass spectrometer equipped with a Knudsen cell will
yield information from emitted species concerning the types of bonds
present and their relative stability. The Knudsen cell has a small hole
in the top which allows vapors, under equilibrium conditions with
the heated sample, to flow into the Bendix time of flight (TOF) mass
spectrometer for analysis.
Ideally, the partial pressure of a constituent follows Raoult’s law
35
which states the partial pressure of species A (P ) is equal to the atomic
A
fraction of A (X ) times the pressure of pure A (P ) at that temperature.
0
A A
Deviation from this value indicates bonding of A in the solid or liquid.
Measuring the pressure of a species as a function of temperature yields
thermodynamic information that can be related to the binding energy
of the species in the solid or liquid phase. The slope of a plot of ln P
A
versus 1/T yields a differential heat of solution for species A in the solid
or liquid. A change in the slope over a temperature range will indicate
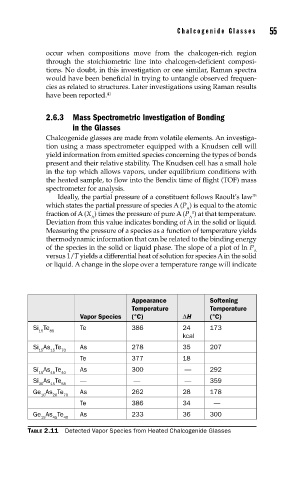
Appearance Softening
Temperature Temperature
Vapor Species (°C) ∆H (°C)
Si Te Te 386 24 173
15 85
kcal
Si As Te As 278 35 207
15 15 70
Te 377 18
Si As Te As 300 — 292
15 45 40
Si As Te — — — 359
30 15 55
Ge As Te As 262 28 178
10 20 70
Te 386 34 —
Ge As Te As 233 36 300
15 45 40
TABLE 2.11 Detected Vapor Species from Heated Chalcogenide Glasses