Page 79 - Chalcogenide Glasses for Infrared Optics
P. 79
Chalcogenide Glasses 57
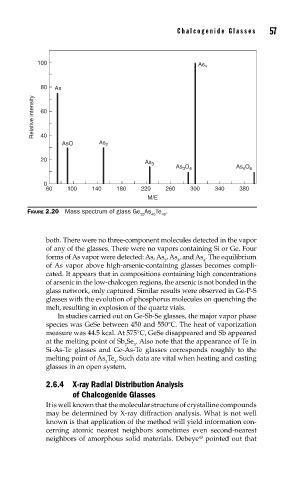
100
As 4
80 As
Relative intensity 60
40
AsO As 2
20
As 3
As 3 O 4 As 4 O 6
0
60 100 140 180 220 260 300 340 380
M/E
FIGURE 2.20 Mass spectrum of glass Ge As Te .
15 45 40
both. There were no three-component molecules detected in the vapor
of any of the glasses. There were no vapors containing Si or Ge. Four
forms of As vapor were detected: As, As , As , and As . The equilibrium
2 3 4
of As vapor above high-arsenic-containing glasses becomes compli-
cated. It appears that in compositions containing high concentrations
of arsenic in the low-chalcogen regions, the arsenic is not bonded in the
glass network, only captured. Similar results were observed in Ge-P-S
glasses with the evolution of phosphorus molecules on quenching the
melt, resulting in explosion of the quartz vials.
In studies carried out on Ge-Sb-Se glasses, the major vapor phase
species was GeSe between 450 and 550°C. The heat of vaporization
measure was 44.5 kcal. At 575°C, GeSe disappeared and Sb appeared
at the melting point of Sb Se . Also note that the appearance of Te in
2 3
Si-As-Te glasses and Ge-As-Te glasses corresponds roughly to the
melting point of As Te .Such data are vital when heating and casting
2 3
glasses in an open system.
2.6.4 X-ray Radial Distribution Analysis
of Chalcogenide Glasses
It is well known that the molecular structure of crystalline compounds
may be determined by X-ray diffraction analysis. What is not well
known is that application of the method will yield information con-
cerning atomic nearest neighbors sometimes even second-nearest
43
neighbors of amorphous solid materials. Debeye pointed out that