Page 83 - Chalcogenide Glasses for Infrared Optics
P. 83
Chalcogenide Glasses 61
To welder power
supply (±)
Glass window
Ar in
1/2 Ar ATM.
Samples
Copper plate W tip
Arc
Ti
Chill water in Water out
H O
2
To welder power
supply (±)
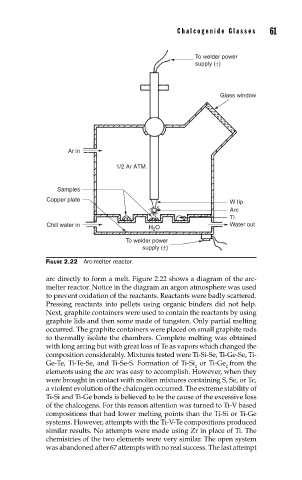
FIGURE 2.22 Arc-melter reactor.
arc directly to form a melt. Figure 2.22 shows a diagram of the arc-
melter reactor. Notice in the diagram an argon atmosphere was used
to prevent oxidation of the reactants. Reactants were badly scattered.
Pressing reactants into pellets using organic binders did not help.
Next, graphite containers were used to contain the reactants by using
graphite lids and then some made of tungsten. Only partial melting
occurred. The graphite containers were placed on small graphite rods
to thermally isolate the chambers. Complete melting was obtained
with long arcing but with great loss of Te as vapors which changed the
composition considerably. Mixtures tested were Ti-Si-Se, Ti-Ge-Se, Ti-
Ge-Te, Ti-Te-Se, and Ti-Se-S. Formation of Ti-Si or Ti-Ge from the
2 2
elements using the arc was easy to accomplish. However, when they
were brought in contact with molten mixtures containing S, Se, or Te,
a violent evolution of the chalcogen occurred. The extreme stability of
Ti-Si and Ti-Ge bonds is believed to be the cause of the excessive loss
of the chalcogens. For this reason attention was turned to Ti-V based
compositions that had lower melting points than the Ti-Si or Ti-Ge
systems. However, attempts with the Ti-V-Te compositions produced
similar results. No attempts were made using Zr in place of Ti. The
chemistries of the two elements were very similar. The open system
was abandoned after 67 attempts with no real success. The last attempt