Page 88 - Chalcogenide Glasses for Infrared Optics
P. 88
66 Cha pte r T w o
Amorphous Crystalline
glass (metallic-like)
solid
(a) Glass outer shell
Amorphous
glass
Crystalline
(metallic-like)
solid
(b) Layered glass
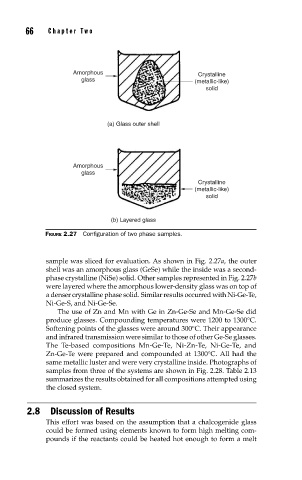
FIGURE 2.27 Confi guration of two phase samples.
sample was sliced for evaluation. As shown in Fig. 2.27a, the outer
shell was an amorphous glass (GeSe) while the inside was a second-
phase crystalline (NiSe) solid. Other samples represented in Fig. 2.27b
were layered where the amorphous lower-density glass was on top of
a denser crystalline phase solid. Similar results occurred with Ni-Ge-Te,
Ni-Ge-S, and Ni-Ge-Se.
The use of Zn and Mn with Ge in Zn-Ge-Se and Mn-Ge-Se did
produce glasses. Compounding temperatures were 1200 to 1300°C.
Softening points of the glasses were around 300°C. Their appearance
and infrared transmission were similar to those of other Ge-Se glasses.
The Te-based compositions Mn-Ge-Te, Ni-Zn-Te, Ni-Ge-Te, and
Zn-Ge-Te were prepared and compounded at 1300°C. All had the
same metallic luster and were very crystalline inside. Photographs of
samples from three of the systems are shown in Fig. 2.28. Table 2.13
summarizes the results obtained for all compositions attempted using
the closed system.
2.8 Discussion of Results
This effort was based on the assumption that a chalcogenide glass
could be formed using elements known to form high melting com-
pounds if the reactants could be heated hot enough to form a melt