Page 87 - Chalcogenide Glasses for Infrared Optics
P. 87
Chalcogenide Glasses 65
It was decided to use elements other than V with Ti to reduce the
melt temperature. Ni and Ge were used along with Se for Te. The
incidence of explosions increased. And still no glasses formed from
homogeneous melts. Compositions containing Ti were abandoned.
Perhaps Ti glasses did not form because the Ti-Te formation broke
down any Te-Te amorphous chain-based structure. The coordination
number for Ti is perhaps 6 or 8 Te atoms at the high melt tempera-
tures. Similar considerations exist for the use of V or Zr. The net result
is that crystallization occurs because of the decrease in viscosity of the
melt. The need for a coordination number of 4 to form glasses is again
supported by the results.
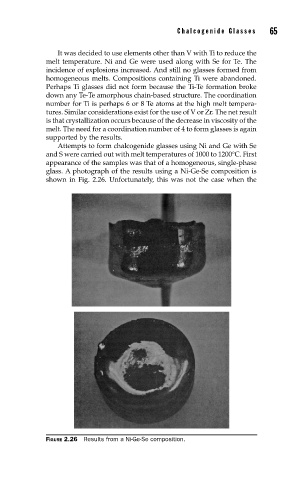
Attempts to form chalcogenide glasses using Ni and Ge with Se
and S were carried out with melt temperatures of 1000 to 1200°C. First
appearance of the samples was that of a homogeneous, single-phase
glass. A photograph of the results using a Ni-Ge-Se composition is
shown in Fig. 2.26. Unfortunately, this was not the case when the
FIGURE 2.26 Results from a Ni-Ge-Se composition.