Page 78 - Chalcogenide Glasses for Infrared Optics
P. 78
56 Cha pte r T w o
a change in bonding within the solid or liquid. So the instrument
identifies the chemical nature of the vapor species, the temperature at
which it appears, and the amount with temperature change. To ensure
the Knudsen cell would function as expected, it was calibrated using
pure arsenic. The value of ∆H obtained for sublimation of arsenic
V
was 31.2 kcal which matches the accepted value of 31 kcal. The tem-
42
perature range of operation was 25 to 500°C, low enough to ensure
equilibrium conditions inside the Knudsen cell were maintained. The
appearance temperature of each species was noted and a heat of
vaporization determined when possible. A summary of results is pre-
sented in Table 2.11.
The heats of vaporization given are based on initial slopes. Note
that in the glass Si As Te there was no vapor species detected up to
30 15 55
500°C, well above its softening point, which is very unusual. The
low-silicon high-tellurium glass showed only tellurium at 386°C, well
above the softening point. The low-As, low-silicon glass gave off As
and Te vapors. The high-As glass emitted As vapor in large amounts.
The high softening Si-As-Te glass gave off no vapors. The high-
tellurium Si-As glass showed As and Te emission. The Ge-As-Te glass
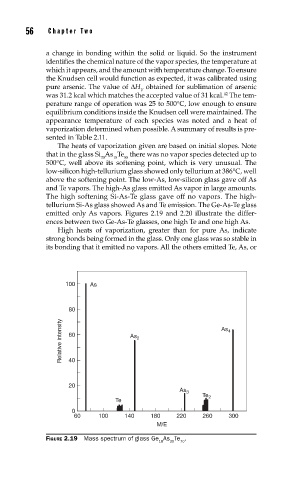
emitted only As vapors. Figures 2.19 and 2.20 illustrate the differ-
ences between two Ge-As-Te glasses, one high Te and one high As.
High heats of vaporization, greater than for pure As, indicate
strong bonds being formed in the glass. Only one glass was so stable in
its bonding that it emitted no vapors. All the others emitted Te, As, or
100 As
80
Relative intensity 60 As 2 As 4
40
20
As 3
Te 2
Te
0
60 100 140 180 220 260 300
M/E
FIGURE 2.19 Mass spectrum of glass Ge As Te .
10 20 70