Page 96 - Chalcogenide Glasses for Infrared Optics
P. 96
74 Cha pte r T h ree
a complete reaction of all constituents. With the quartz chamber
under vacuum, reaching the boiling point of the chalcogen offsets the
atmospheric pressure from outside. Each glass composition is different
relative to internal pressure and heating. Care must be taken to avoid
excessive internal pressure and quartz failure with the resulting reac-
tion of the molten glass with the atmospheric oxygen. Compounding
should be carried out in a closed room or hood with provisions made
for exhausting any fumes in case of quartz failure.
After the reaction period is over, while still rocking, the temperature
is lowered to a point well above the softening point of the glass where
the melt is still fluid. At this point, the glass is ready to be quenched.
The furnace is placed in a near-vertical position. Prior arrangements
have been made to prevent the tube of glass from falling from the fur-
nace. Room air or blown air comes in contact with the surface of the tube.
The temperature is monitored to ensure the glass reaches and stays well
below T After the furnace has cooled, the glass may be left to cool in
g.
room air. After cooling is complete, the quartz tube is vented to air. The
quartz tube is carefully fractured and removed from the glass. The result
of the operation is a 1- to 2-kg cylinder, sometimes called a boule, of the
specified glass composition ready for evaluation.
3.3 Compounding with Reactant Purification
In the early period of producing chalcogenide glasses at TI, the rocking
furnace method described was used. In the early 1970s, the U.S. Air Force
launched a great effort dedicated to developing large windows for high-
energy CO lasers. TI became involved because in its active program
2
large glass windows had already been cast. Reduction of absorption at
10.6 µm was a top priority. Examination of the glass using an infrared
microscope identified the existence of particulate matter in the glass that
2,3
turned out to be carbon from the selenium. An effort was made to
remove all impurities and oxides by distillation using filters to establish
the intrinsic transmission for TI 1173 and TI 20 glasses. The apparatus
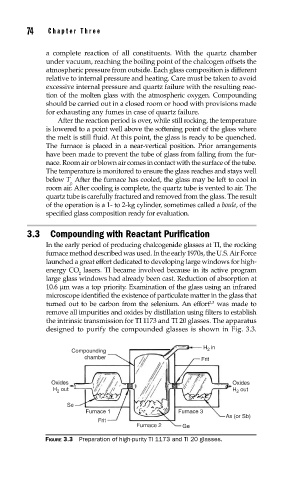
designed to purify the compounded glasses is shown in Fig. 3.3.
H 2 in
Compounding
chamber Frit
Oxides Oxides
H 2 out H 2 out
Se
Furnace 1 Furnace 3
As (or Sb)
Frit
Furnace 2 Ge
FIGURE 3.3 Preparation of high-purity TI 1173 and TI 20 glasses.