Page 417 - Chemical process engineering design and economics
P. 417
Reactor Design 397
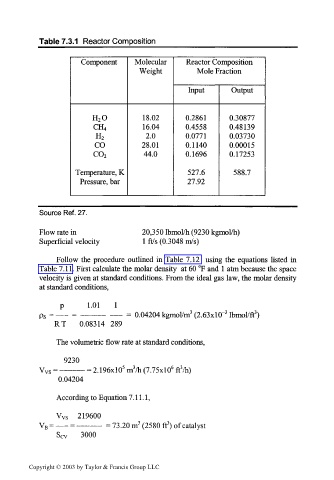
Table 7.3.1 Reactor Composition
Component Molecular Reactor Composition
Weight Mole Fraction
Input Output
H 2O 18.02 0.2861 0.30877
16.04 0.4558 0.48139
CH 4
2.0 0.0771 0.03730
H 2
CO 28.01 0.1140 0.00015
44.0 0.1696 0.17253
C0 2
Temperature, K 527.6 588.7
Pressure, bar 27.92
Source Ref. 27.
Flow rate in 20,350 Ibmol/h (9230 kgmol/h)
Superficial velocity 1 fl/s (0.3048 m/s)
Follow the procedure outlined in Table 7.12 using the equations listed in
Table 7.11. First calculate the molar density at 60 °F and 1 ami because the space
velocity is given at standard conditions. From the ideal gas law, the molar density
at standard conditions,
1.01 1
3
3
3
Ps — ——— —— = 0.04204 kgmol/m (2.63x10~ lbmol/ft )
RT 0.08314 289
The volumetric flow rate at standard conditions,
9230
6
3
5
V vs = ———— = 2.196xl0 m /h (7.75xl0 ftVh)
0.04204
According to Equation 7.11.1,
V vs 219600
3
3
V B = —— = ———— = 73.20 m (2580 ft ) of catalyst
3000
S cv
Copyright © 2003 by Taylor & Francis Group LLC