Page 191 - Control Theory in Biomedical Engineering
P. 191
Medical robotics 175
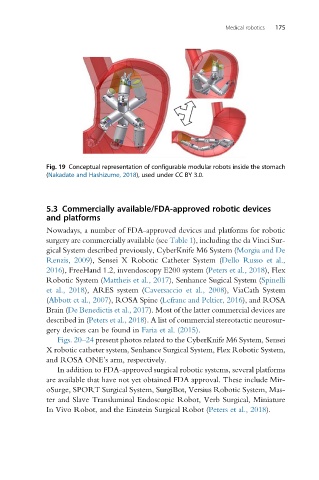
Fig. 19 Conceptual representation of configurable modular robots inside the stomach
(Nakadate and Hashizume, 2018), used under CC BY 3.0.
5.3 Commercially available/FDA-approved robotic devices
and platforms
Nowadays, a number of FDA-approved devices and platforms for robotic
surgery are commercially available (see Table 1), including the da Vinci Sur-
gical System described previously, CyberKnife M6 System (Morgia and De
Renzis, 2009), Sensei X Robotic Catheter System (Dello Russo et al.,
2016), FreeHand 1.2, invendoscopy E200 system (Peters et al., 2018), Flex
Robotic System (Mattheis et al., 2017), Senhance Sugical System (Spinelli
et al., 2018), ARES system (Caversaccio et al., 2008), ViaCath System
(Abbott et al., 2007), ROSA Spine (Lefranc and Peltier, 2016), and ROSA
Brain (De Benedictis et al., 2017). Most of the latter commercial devices are
described in (Peters et al., 2018). A list of commercial stereotactic neurosur-
gery devices can be found in Faria et al. (2015).
Figs. 20–24 present photos related to the CyberKnife M6 System, Sensei
X robotic catheter system, Senhance Surgical System, Flex Robotic System,
and ROSA ONE’s arm, respectively.
In addition to FDA-approved surgical robotic systems, several platforms
are available that have not yet obtained FDA approval. These include Mir-
oSurge, SPORT Surgical System, SurgiBot, Versius Robotic System, Mas-
ter and Slave Transluminal Endoscopic Robot, Verb Surgical, Miniature
In Vivo Robot, and the Einstein Surgical Robot (Peters et al., 2018).