Page 191 - Corrosion Engineering Principles and Practice
P. 191
166 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 167
Cathode
Metal
e –
H +
Corrosion products
Anode
(c)
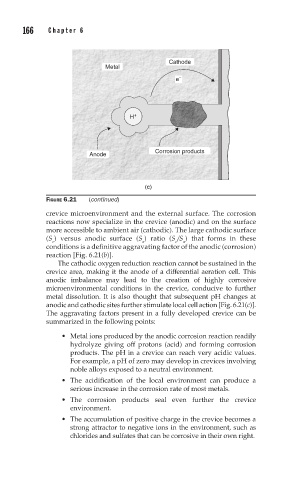
FIGURE 6.21 (continued)
crevice microenvironment and the external surface. The corrosion
reactions now specialize in the crevice (anodic) and on the surface
more accessible to ambient air (cathodic). The large cathodic surface
(S ) versus anodic surface (S ) ratio (S /S ) that forms in these
a
c
c
a
conditions is a definitive aggravating factor of the anodic (corrosion)
reaction [Fig. 6.21(b)].
The cathodic oxygen reduction reaction cannot be sustained in the
crevice area, making it the anode of a differential aeration cell. This
anodic imbalance may lead to the creation of highly corrosive
microenvironmental conditions in the crevice, conducive to further
metal dissolution. It is also thought that subsequent pH changes at
anodic and cathodic sites further stimulate local cell action [Fig. 6.21(c)].
The aggravating factors present in a fully developed crevice can be
summarized in the following points:
• Metal ions produced by the anodic corrosion reaction readily
hydrolyze giving off protons (acid) and forming corrosion
products. The pH in a crevice can reach very acidic values.
For example, a pH of zero may develop in crevices involving
noble alloys exposed to a neutral environment.
• The acidification of the local environment can produce a
serious increase in the corrosion rate of most metals.
• The corrosion products seal even further the crevice
environment.
• The accumulation of positive charge in the crevice becomes a
strong attractor to negative ions in the environment, such as
chlorides and sulfates that can be corrosive in their own right.