Page 192 - Corrosion Engineering Principles and Practice
P. 192
166 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 167
An Ideal Crevice
Many mathematical models have been developed to simulate the
initiation and propagation of crevice corrosion processes as a function
of external electrolyte composition and potential. Such models are
deemed to be quite important for predicting the behavior of otherwise
benign situations that can progress into serious corrosion situations.
One such model was applied to several experimental datasets,
including crevice corrosion initiation on stainless steel and active
corrosion of iron in several electrolytes [11]. The model was said to
break new ground by
• Using equations for moderately concentrated solutions and
including individual ion-activity coefficients. Transport by
chemical potential gradients was used rather than equations
for dilute solutions.
• Being capable of handling passive corrosion, active corrosion,
and active/passive transitions in transient systems.
• Being generic and permitting the evaluation of the importance
of different species, chemical reactions, metals, and types of
kinetics at the metal/solution interface.
Solution of the model for a particular problem requires specifica-
tion of the chemical species considered, their respective possible reac-
tions, supporting thermodynamic data, grid geometry, and kinetics
at the metal/solution interface. The simulation domain is then bro-
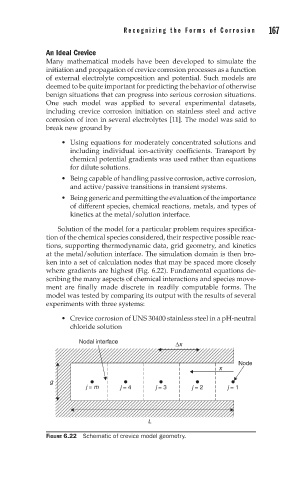
ken into a set of calculation nodes that may be spaced more closely
where gradients are highest (Fig. 6.22). Fundamental equations de-
scribing the many aspects of chemical interactions and species move-
ment are finally made discrete in readily computable forms. The
model was tested by comparing its output with the results of several
experiments with three systems:
• Crevice corrosion of UNS 30400 stainless steel in a pH-neutral
chloride solution
Nodal interface
∆x
Node
x
g
j = m j = 4 j = 3 j = 2 j = 1
L
FIGURE 6.22 Schematic of crevice model geometry.