Page 196 - Corrosion Engineering Principles and Practice
P. 196
170 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 171
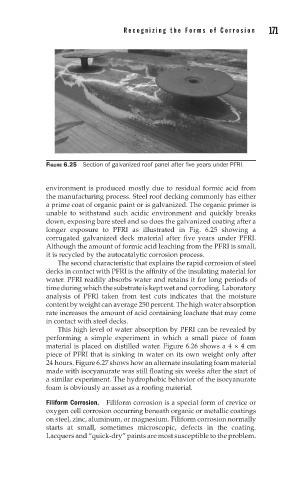
FIGURE 6.25 Section of galvanized roof panel after five years under PFRI.
environment is produced mostly due to residual formic acid from
the manufacturing process. Steel roof decking commonly has either
a prime coat of organic paint or is galvanized. The organic primer is
unable to withstand such acidic environment and quickly breaks
down, exposing bare steel and so does the galvanized coating after a
longer exposure to PFRI as illustrated in Fig. 6.25 showing a
corrugated galvanized deck material after five years under PFRI.
Although the amount of formic acid leaching from the PFRI is small,
it is recycled by the autocatalytic corrosion process.
The second characteristic that explains the rapid corrosion of steel
decks in contact with PFRI is the affinity of the insulating material for
water. PFRI readily absorbs water and retains it for long periods of
time during which the substrate is kept wet and corroding. Laboratory
analysis of PFRI taken from test cuts indicates that the moisture
content by weight can average 250 percent. The high water absorption
rate increases the amount of acid containing leachate that may come
in contact with steel decks.
This high level of water absorption by PFRI can be revealed by
performing a simple experiment in which a small piece of foam
material is placed on distilled water. Figure 6.26 shows a 4 × 4 cm
piece of PFRI that is sinking in water on its own weight only after
24 hours. Figure 6.27 shows how an alternate insulating foam material
made with isocyanurate was still floating six weeks after the start of
a similar experiment. The hydrophobic behavior of the isocyanurate
foam is obviously an asset as a roofing material.
Filiform Corrosion. Filiform corrosion is a special form of crevice or
oxygen cell corrosion occurring beneath organic or metallic coatings
on steel, zinc, aluminum, or magnesium. Filiform corrosion normally
starts at small, sometimes microscopic, defects in the coating.
Lacquers and “quick-dry” paints are most susceptible to the problem.