Page 195 - Corrosion Engineering Principles and Practice
P. 195
170 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 171
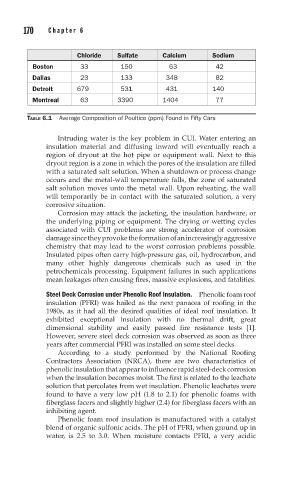
Chloride Sulfate Calcium Sodium
Boston 33 150 63 42
Dallas 23 133 348 82
Detroit 679 531 431 140
Montreal 63 3390 1404 77
TABLE 6.1 Average Composition of Poultice (ppm) Found in Fifty Cars
Intruding water is the key problem in CUI. Water entering an
insulation material and diffusing inward will eventually reach a
region of dryout at the hot pipe or equipment wall. Next to this
dryout region is a zone in which the pores of the insulation are filled
with a saturated salt solution. When a shutdown or process change
occurs and the metal-wall temperature falls, the zone of saturated
salt solution moves unto the metal wall. Upon reheating, the wall
will temporarily be in contact with the saturated solution, a very
corrosive situation.
Corrosion may attack the jacketing, the insulation hardware, or
the underlying piping or equipment. The drying or wetting cycles
associated with CUI problems are strong accelerator of corrosion
damage since they provoke the formation of an increasingly aggressive
chemistry that may lead to the worst corrosion problems possible.
Insulated pipes often carry high-pressure gas, oil, hydrocarbon, and
many other highly dangerous chemicals such as used in the
petrochemicals processing. Equipment failures in such applications
mean leakages often causing fires, massive explosions, and fatalities.
Steel Deck Corrosion under Phenolic Roof Insulation. Phenolic foam roof
insulation (PFRI) was hailed as the next panacea of roofing in the
1980s, as it had all the desired qualities of ideal roof insulation. It
exhibited exceptional insulation with no thermal drift, great
dimensional stability and easily passed fire resistance tests [1].
However, severe steel deck corrosion was observed as soon as three
years after commercial PFRI was installed on some steel decks.
According to a study performed by the National Roofing
Contractors Association (NRCA), there are two characteristics of
phenolic insulation that appear to influence rapid steel-deck corrosion
when the insulation becomes moist. The first is related to the leachate
solution that percolates from wet insulation. Phenolic leachates were
found to have a very low pH (1.8 to 2.1) for phenolic foams with
fiberglass facers and slightly higher (2.4) for fiberglass facers with an
inhibiting agent.
Phenolic foam roof insulation is manufactured with a catalyst
blend of organic sulfonic acids. The pH of PFRI, when ground up in
water, is 2.5 to 3.0. When moisture contacts PFRI, a very acidic