Page 198 - Corrosion Engineering Principles and Practice
P. 198
172 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 173
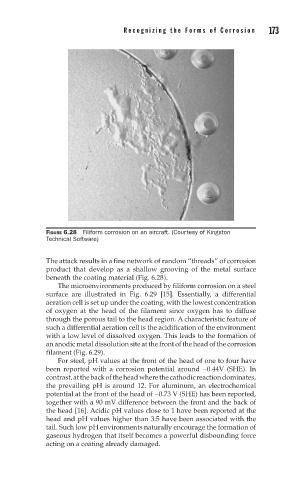
FIGURE 6.28 Filiform corrosion on an aircraft. (Courtesy of Kingston
Technical Software)
The attack results in a fine network of random “threads” of corrosion
product that develop as a shallow grooving of the metal surface
beneath the coating material (Fig. 6.28).
The microenvironments produced by filiform corrosion on a steel
surface are illustrated in Fig. 6.29 [15]. Essentially, a differential
aeration cell is set up under the coating, with the lowest concentration
of oxygen at the head of the filament since oxygen has to diffuse
through the porous tail to the head region. A characteristic feature of
such a differential aeration cell is the acidification of the environment
with a low level of dissolved oxygen. This leads to the formation of
an anodic metal dissolution site at the front of the head of the corrosion
filament (Fig. 6.29).
For steel, pH values at the front of the head of one to four have
been reported with a corrosion potential around −0.44V (SHE). In
contrast, at the back of the head where the cathodic reaction dominates,
the prevailing pH is around 12. For aluminum, an electrochemical
potential at the front of the head of −0.73 V (SHE) has been reported,
together with a 90 mV difference between the front and the back of
the head [16]. Acidic pH values close to 1 have been reported at the
head and pH values higher than 3.5 have been associated with the
tail. Such low pH environments naturally encourage the formation of
gaseous hydrogen that itself becomes a powerful disbounding force
acting on a coating already damaged.