Page 203 - Corrosion Engineering Principles and Practice
P. 203
178 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 179
(a)
(b)
(c)
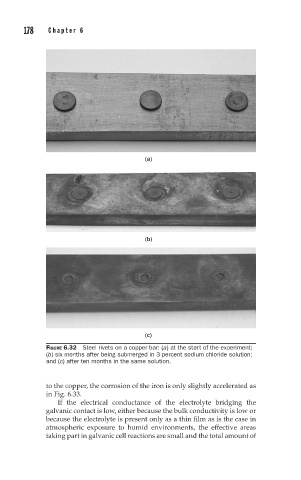
FIGURE 6.32 Steel rivets on a copper bar: (a) at the start of the experiment;
(b) six months after being submerged in 3 percent sodium chloride solution;
and (c) after ten months in the same solution.
to the copper, the corrosion of the iron is only slightly accelerated as
in Fig. 6.33.
If the electrical conductance of the electrolyte bridging the
galvanic contact is low, either because the bulk conductivity is low or
because the electrolyte is present only as a thin film as is the case in
atmospheric exposure to humid environments, the effective areas
taking part in galvanic cell reactions are small and the total amount of