Page 204 - Corrosion Engineering Principles and Practice
P. 204
178 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 179
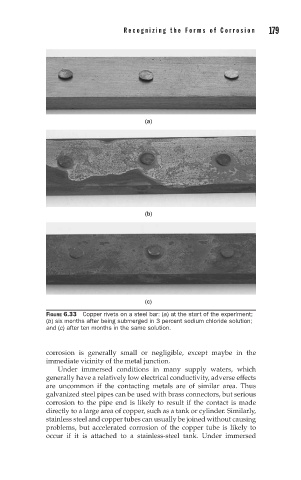
(a)
(b)
(c)
FIGURE 6.33 Copper rivets on a steel bar: (a) at the start of the experiment;
(b) six months after being submerged in 3 percent sodium chloride solution;
and (c) after ten months in the same solution.
corrosion is generally small or negligible, except maybe in the
immediate vicinity of the metal junction.
Under immersed conditions in many supply waters, which
generally have a relatively low electrical conductivity, adverse effects
are uncommon if the contacting metals are of similar area. Thus
galvanized steel pipes can be used with brass connectors, but serious
corrosion to the pipe end is likely to result if the contact is made
directly to a large area of copper, such as a tank or cylinder. Similarly,
stainless steel and copper tubes can usually be joined without causing
problems, but accelerated corrosion of the copper tube is likely to
occur if it is attached to a stainless-steel tank. Under immersed