Page 205 - Corrosion Engineering Principles and Practice
P. 205
180 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 181
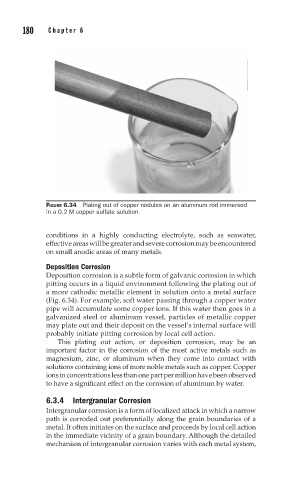
FIGURE 6.34 Plating out of copper nodules on an aluminum rod immersed
in a 0.2 M copper sulfate solution.
conditions in a highly conducting electrolyte, such as seawater,
effective areas will be greater and severe corrosion may be encountered
on small anodic areas of many metals.
Deposition Corrosion
Deposition corrosion is a subtle form of galvanic corrosion in which
pitting occurs in a liquid environment following the plating out of
a more cathodic metallic element in solution onto a metal surface
(Fig. 6.34). For example, soft water passing through a copper water
pipe will accumulate some copper ions. If this water then goes in a
galvanized steel or aluminum vessel, particles of metallic copper
may plate out and their deposit on the vessel’s internal surface will
probably initiate pitting corrosion by local cell action.
This plating out action, or deposition corrosion, may be an
important factor in the corrosion of the most active metals such as
magnesium, zinc, or aluminum when they come into contact with
solutions containing ions of more noble metals such as copper. Copper
ions in concentrations less than one part per million have been observed
to have a significant effect on the corrosion of aluminum by water.
6.3.4 Intergranular Corrosion
Intergranular corrosion is a form of localized attack in which a narrow
path is corroded out preferentially along the grain boundaries of a
metal. It often initiates on the surface and proceeds by local cell action
in the immediate vicinity of a grain boundary. Although the detailed
mechanism of intergranular corrosion varies with each metal system,