Page 209 - Corrosion Engineering Principles and Practice
P. 209
184 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 185
The HIC mechanism has not yet fully established. Various factors
are believed to contribute to unlocking the lattice of the metal, such as
hydrogen pressure at the crack tip, the competition of hydrogen
atoms for the lattice-bonding electrons, easier plastic flow of
dislocations in the metal at the crack tip in the presence of hydrogen,
and the formation of certain metal hydrides in the alloy.
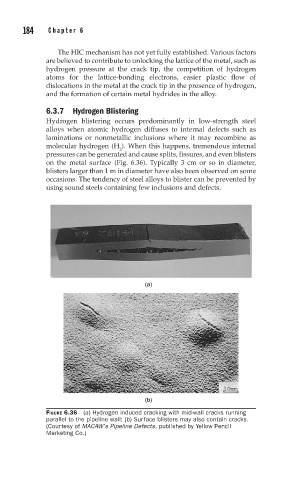
6.3.7 Hydrogen Blistering
Hydrogen blistering occurs predominantly in low-strength steel
alloys when atomic hydrogen diffuses to internal defects such as
laminations or nonmetallic inclusions where it may recombine as
molecular hydrogen (H ). When this happens, tremendous internal
2
pressures can be generated and cause splits, fissures, and even blisters
on the metal surface (Fig. 6.36). Typically 3 cm or so in diameter,
blisters larger than 1 m in diameter have also been observed on some
occasions. The tendency of steel alloys to blister can be prevented by
using sound steels containing few inclusions and defects.
(a)
(b)
FIGURE 6.36 (a) Hydrogen induced cracking with mid-wall cracks running
parallel to the pipeline wall; (b) Surface blisters may also contain cracks.
(Courtesy of MACAW’s Pipeline Defects, published by Yellow Pencil
Marketing Co.)