Page 207 - Corrosion Engineering Principles and Practice
P. 207
182 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 183
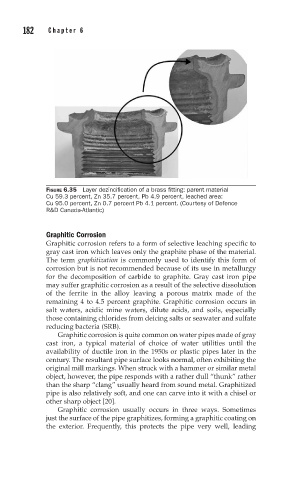
FIGURE 6.35 Layer dezincification of a brass fitting: parent material
Cu 59.3 percent, Zn 35.7 percent, Pb 4.9 percent, leached area:
Cu 95.0 percent, Zn 0.7 percent Pb 4.1 percent. (Courtesy of Defence
R&D Canada-Atlantic)
Graphitic Corrosion
Graphitic corrosion refers to a form of selective leaching specific to
gray cast iron which leaves only the graphite phase of the material.
The term graphitization is commonly used to identify this form of
corrosion but is not recommended because of its use in metallurgy
for the decomposition of carbide to graphite. Gray cast iron pipe
may suffer graphitic corrosion as a result of the selective dissolution
of the ferrite in the alloy leaving a porous matrix made of the
remaining 4 to 4.5 percent graphite. Graphitic corrosion occurs in
salt waters, acidic mine waters, dilute acids, and soils, especially
those containing chlorides from deicing salts or seawater and sulfate
reducing bacteria (SRB).
Graphitic corrosion is quite common on water pipes made of gray
cast iron, a typical material of choice of water utilities until the
availability of ductile iron in the 1950s or plastic pipes later in the
century. The resultant pipe surface looks normal, often exhibiting the
original mill markings. When struck with a hammer or similar metal
object, however, the pipe responds with a rather dull “thunk” rather
than the sharp “clang” usually heard from sound metal. Graphitized
pipe is also relatively soft, and one can carve into it with a chisel or
other sharp object [20].
Graphitic corrosion usually occurs in three ways. Sometimes
just the surface of the pipe graphitizes, forming a graphitic coating on
the exterior. Frequently, this protects the pipe very well, leading