Page 199 - Corrosion Engineering Principles and Practice
P. 199
174 C h a p t e r 6 R e c o g n i z i n g t h e F o r m s o f C o r r o s i o n 175
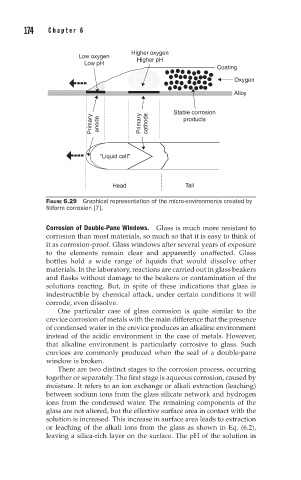
Higher oxygen
Low oxygen
Low pH Higher pH
Coating
Oxygen
Alloy
Stable corrosion
Primary anode Primary cathode products
“Liquid cell’’
Head Tail
FIGURE 6.29 Graphical representation of the micro-environments created by
filiform corrosion [7].
Corrosion of Double-Pane Windows. Glass is much more resistant to
corrosion than most materials, so much so that it is easy to think of
it as corrosion-proof. Glass windows after several years of exposure
to the elements remain clear and apparently unaffected. Glass
bottles hold a wide range of liquids that would dissolve other
materials. In the laboratory, reactions are carried out in glass beakers
and flasks without damage to the beakers or contamination of the
solutions reacting. But, in spite of these indications that glass is
indestructible by chemical attack, under certain conditions it will
corrode, even dissolve.
One particular case of glass corrosion is quite similar to the
crevice corrosion of metals with the main difference that the presence
of condensed water in the crevice produces an alkaline environment
instead of the acidic environment in the case of metals. However,
that alkaline environment is particularly corrosive to glass. Such
crevices are commonly produced when the seal of a double-pane
window is broken.
There are two distinct stages to the corrosion process, occurring
together or separately. The first stage is aqueous corrosion, caused by
moisture. It refers to an ion exchange or alkali extraction (leaching)
between sodium ions from the glass silicate network and hydrogen
ions from the condensed water. The remaining components of the
glass are not altered, but the effective surface area in contact with the
solution is increased. This increase in surface area leads to extraction
or leaching of the alkali ions from the glass as shown in Eq. (6.2),
leaving a silica-rich layer on the surface. The pH of the solution in