Page 348 - Corrosion Engineering Principles and Practice
P. 348
318 C h a p t e r 8 C o r r o s i o n b y W a t e r 319
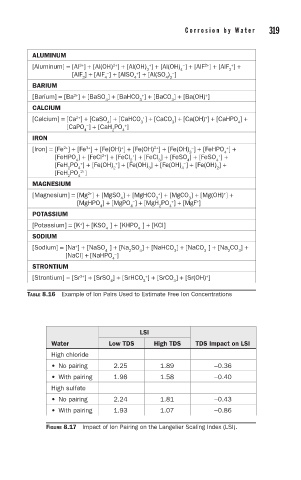
ALUMINUM
2+
−
+
+
3+
2+
[Aluminum] = [Al ] + [Al(OH) ] + [Al(OH) ] + [Al(OH) ] + [AlF ] + [AlF ] +
4
2
2
[AlF ] + [AlF ] + [AlSO ] + [Al(SO ) ]
−
+
−
3 4 4 4 2
BARIUM
2+
+
[Barium] = [Ba ] + [BaSO ] + [BaHCO ] + [BaCO ] + [Ba(OH) ]
+
4 3 3
CALCIUM
[
2+
+
+
[Calcium] = Ca ] + [CaSO ] + [CaHCO ] + [CaCO ] + [Ca(OH) ] + [CaHPO ] +
3
4
4
3
+
−
[CaPO ] + [CaH PO ]
4 2 4
IRON
−
2+
3+
2+
+
+
[Iron] = [Fe ] + [Fe ] + [Fe(OH) ] + [Fe(OH) ] + [Fe(OH) ] + [FeHPO ] +
3
4
+
[FeHPO ] + [FeCl ] + [FeCl ] + [FeCl ] + [FeSO ] + [FeSO ] +
2+
+
4
4
2
3
4
+
−
+
[FeH PO ] + [Fe(OH) ] + [Fe(OH) ] + [Fe(OH) ] + [Fe(OH) ] +
4
2
3
2
2
4
[FeH PO 2+ ]
2 4
MAGNESIUM
2+
[Magnesium] = [Mg ] + [MgSO ] + [MgHCO ] + [MgCO ] + [Mg(OH) ] +
+
+
3
3
4
+
+
[MgHPO ] + [MgPO ] + [MgH PO ] + [MgF ]
−
4 4 2 4
POTASSIUM
+
[Potassium] = [K ] + [KSO ] + [KHPO ] + [KCl]
−
−
4 4
SODIUM
−
−
+
[Sodium] = [Na ] + [NaSO ] + [Na SO ] + [NaHCO ] + [NaCO ] + [Na CO ] +
3
4
3
3
2
4
2
[NaCl] + [NaHPO ]
−
4
STRONTIUM
2+
[Strontium] = [Sr ] + [SrSO ] + [SrHCO ] + [SrCO ] + [Sr(OH) ]
+
+
4 3 3
TABLE 8.16 Example of Ion Pairs Used to Estimate Free Ion Concentrations
LSI
Water Low TDS High TDS TDS Impact on LSI
High chloride
• No pairing 2.25 1.89 −0.36
• With pairing 1.98 1.58 −0.40
High sulfate
• No pairing 2.24 1.81 −0.43
• With pairing 1.93 1.07 −0.86
FIGURE 8.17 Impact of Ion Pairing on the Langelier Scaling Index (LSI).