Page 351 - Corrosion Engineering Principles and Practice
P. 351
322 C h a p t e r 8 C o r r o s i o n b y W a t e r 323
2.5
2
Degree of saturation 1.5 1
0.5 0
17
33
50
0 67 % Injection
25 83
42 58 75 100
Temperature 92 108 125
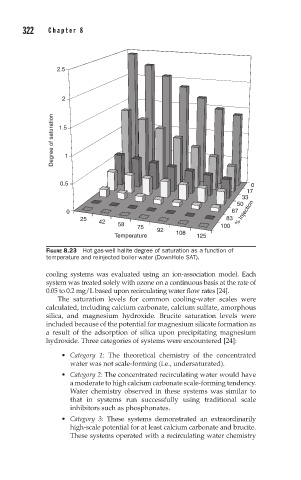
FIGURE 8.23 Hot gas-well halite degree of saturation as a function of
temperature and reinjected boiler water (DownHole SAT).
cooling systems was evaluated using an ion-association model. Each
system was treated solely with ozone on a continuous basis at the rate of
0.05 to 0.2 mg/L based upon recirculating water flow rates [24].
The saturation levels for common cooling-water scales were
calculated, including calcium carbonate, calcium sulfate, amorphous
silica, and magnesium hydroxide. Brucite saturation levels were
included because of the potential for magnesium silicate formation as
a result of the adsorption of silica upon precipitating magnesium
hydroxide. Three categories of systems were encountered [24]:
• Category 1: The theoretical chemistry of the concentrated
water was not scale-forming (i.e., undersaturated).
• Category 2: The concentrated recirculating water would have
a moderate to high calcium carbonate scale-forming tendency.
Water chemistry observed in these systems was similar to
that in systems run successfully using traditional scale
inhibitors such as phosphonates.
• Category 3: These systems demonstrated an extraordinarily
high-scale potential for at least calcium carbonate and brucite.
These systems operated with a recirculating water chemistry