Page 349 - Corrosion Engineering Principles and Practice
P. 349
320 C h a p t e r 8 C o r r o s i o n b y W a t e r 321
concentrations has been used successfully as the basis for developing
models which describe the minimum effective scale inhibitor dosage
that will maintain clean heat-transfer surfaces [23]. The following
cases illustrate some practical usage of the ion-association model.
8.8.1 Limiting Halite Deposition in a Wet
High-Temperature Gas Well
There are several gas-well fields that produce hydrocarbon gas
associated with very high TDS connate waters. Classical oilfield scale
problems (e.g., calcium carbonate, barium sulfate, and calcium
sulfate) are minimal in these fields. Halite (NaCl), however, can be
precipitated to such an extent that production is lost in hours. As a
result, a bottom-hole fluid sample is retrieved from all new wells.
Unstable components are “fixed” immediately after sampling, and
pH is determined under pressure. A full ionic and physical analysis is
also carried out in the laboratory.
Some of the analyses were run through an ion-association model
computer program to determine the susceptibility of the brine to halite
precipitation. If a halite precipitation problem was predicted, the ion-
association model was run in a “mixing” mode to determine if mixing the
connate water with boiler feedwater would prevent the problem. This
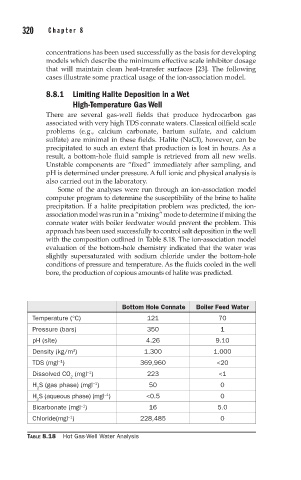
approach has been used successfully to control salt deposition in the well
with the composition outlined in Table 8.18. The ion-association model
evaluation of the bottom-hole chemistry indicated that the water was
slightly supersaturated with sodium chloride under the bottom-hole
conditions of pressure and temperature. As the fluids cooled in the well
bore, the production of copious amounts of halite was predicted.
Bottom Hole Connate Boiler Feed Water
Temperature (°C) 121 70
Pressure (bars) 350 1
pH (site) 4.26 9.10
Density (kg/m ) 1.300 1.000
3
−1
TDS (mgl ) 369,960 <20
−1
Dissolved CO (mgl ) 223 <1
2
H S (gas phase) (mgl ) 50 0
−1
2
H S (aqueous phase) (mgl ) <0.5 0
−1
2
Bicarbonate (mgl ) 16 5.0
−1
−1
Chloride(mgl ) 228,485 0
TABLE 8.18 Hot Gas-Well Water Analysis