Page 350 - Corrosion Engineering Principles and Practice
P. 350
320 C h a p t e r 8 C o r r o s i o n b y W a t e r 321
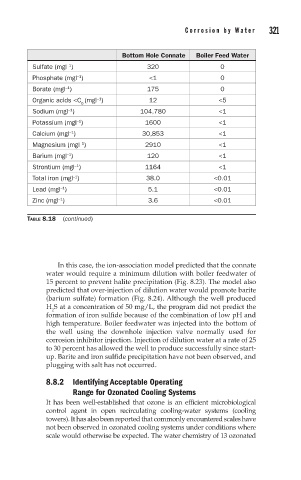
Bottom Hole Connate Boiler Feed Water
−1
Sulfate (mgl ) 320 0
Phosphate (mgl ) <1 0
−1
Borate (mgl ) 175 0
−1
−1
Organic acids <C (mgl ) 12 <5
6
Sodium (mgl ) 104,780 <1
−1
Potassium (mgl ) 1600 <1
−1
Calcium (mgl ) 30,853 <1
−1
Magnesium (mgl ) 2910 <1
−1
−1
Barium (mgl ) 120 <1
Strontium (mgl ) 1164 <1
−1
Total iron (mgl ) 38.0 <0.01
−1
Lead (mgl ) 5.1 <0.01
−1
Zinc (mgl ) 3.6 <0.01
−1
TABLE 8.18 (continued)
In this case, the ion-association model predicted that the connate
water would require a minimum dilution with boiler feedwater of
15 percent to prevent halite precipitation (Fig. 8.23). The model also
predicted that over-injection of dilution water would promote barite
(barium sulfate) formation (Fig. 8.24). Although the well produced
H S at a concentration of 50 mg/L, the program did not predict the
2
formation of iron sulfide because of the combination of low pH and
high temperature. Boiler feedwater was injected into the bottom of
the well using the downhole injection valve normally used for
corrosion inhibitor injection. Injection of dilution water at a rate of 25
to 30 percent has allowed the well to produce successfully since start-
up. Barite and iron sulfide precipitation have not been observed, and
plugging with salt has not occurred.
8.8.2 Identifying Acceptable Operating
Range for Ozonated Cooling Systems
It has been well-established that ozone is an efficient microbiological
control agent in open recirculating cooling-water systems (cooling
towers). It has also been reported that commonly encountered scales have
not been observed in ozonated cooling systems under conditions where
scale would otherwise be expected. The water chemistry of 13 ozonated